Exploring respiratory support options with Dr. Adam Seiver
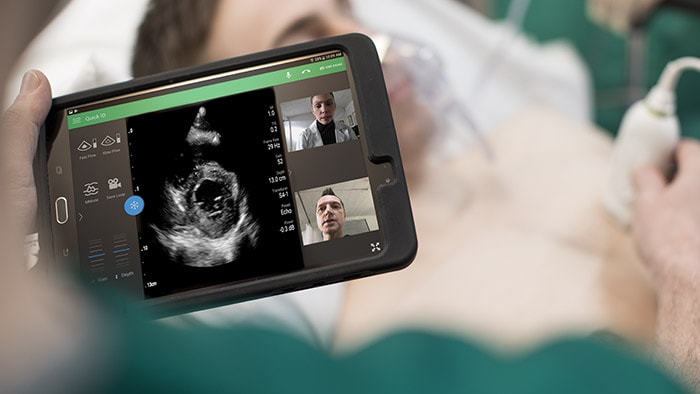
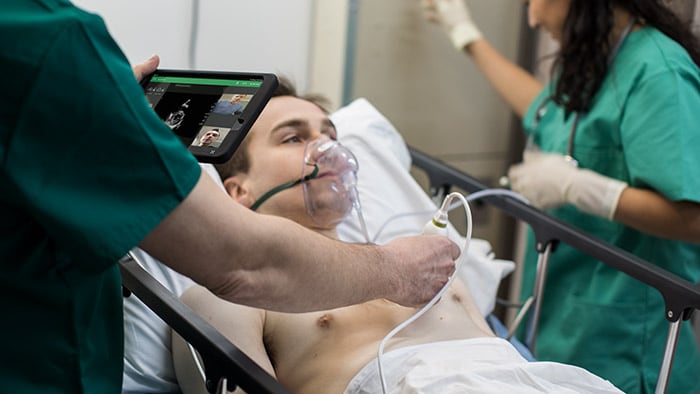
At the American Thoracic Society’s ATS 2020 Virtual event, care providers from around the world gathered to discuss the latest advances in research, patient care, and public health in pulmonary disease, critical illness, and sleep disorders. This year, the conference would have been remiss without addressing the COVID-19 crisis, which has affected every aspect of healthcare across the world. And, while we don’t yet know the full extent of the pandemic’s impacts, we do know that they will be lasting. Our own Dr. Adam Seiver, Medical Leader, Therapeutic Care and Hospital Respiratory Care at Philips, gave a virtual symposium at ATS 2020 on the different types of ventilatory support options for COVID-19 patients. Before the event, we sat down with Dr. Seiver to learn more about these therapy options, along with his insights on the pandemic, and his advice for managing the virus in the months to come. Continue reading to learn more. Q: For COVID-19 patients in respiratory distress, what treatment options are there? A: When we assess patients in respiratory distress, they’re viewed across a continuum of acute respiratory failure – from very mild, which may only require low oxygen flow therapy, to very severe, in which case patients are intubated and placed on a ventilator. To treat the needs of this continuum of patients, clinicians have a breadth of therapies in their respiratory toolbox to consider, such as high flow oxygen therapy and non-invasive ventilation, as well as invasive ventilation. For COVID-19 patients in particular, we’ve seen clinicians frequently use high flow oxygen therapy as well as non-invasive ventilation to treat acute respiratory failure. Q: Can you explain the difference between non-invasive ventilation and high flow therapy? How should care providers prescribe these treatments in response to COVID-19? A: Non-invasive ventilation, often referred to as NIV, is the administration of respiratory support with a mask instead of an invasive artificial airway (endotracheal tube) to deliver oxygen. This therapy has been widely accepted for years for the treatment of patients in acute respiratory distress, both at home and in the hospital. High flow oxygen therapy, or HFOT, is another respiratory therapy in which oxygen is delivered to a patient at higher flows than traditional oxygen therapy. In terms of treating COVID-19 patients, if a patient can manage their symptoms at home and these symptoms are not worsening, we recommend that they stay home. But for those that are experiencing enough respiratory distress that they need additional medical help, we have seen success with using both NIV and HFOT. Here, there are not definitive studies yet on the type of treatment that is best to use for COVID-19 patients. What’s important is delivering a therapy that best balances the risks and benefits for the individual patient, while respecting and mitigating risks to the caregiver. In many cases, HFOT or NIV may be best. Q: In the COVID-19 toolkit you referenced, where does the E30 come into play? A: When we first started to see a rise of COVID-19 cases in the United States, there was a massive shift in the health technology industry to exponentially increase the production of ventilators. At Philips, we were able to take our continuous positive airway pressure (CPAP) machines – traditionally prescribed as an at-home treatment for sleep apnea patients –and convert them into hospital grade ventilators. This innovation allowed us to start producing thousands of ventilators per week. To be honest, this was one of the most exciting projects that I have ever worked on. One of the greatest things about the E30 [1] is its multifunctionality – it can be used as both an invasive and non-invasive ventilator. In terms of the COVID-19 toolkit, it is valuable to have a multi-use asset that can be used across the spectrum of respiratory failure as a patient trajectory evolves. Q: How have COVID-19 treatments evolved? Do you see them evolving further as we learn more about the disease? A: Early in the pandemic, there was a pervasive opinion that non-invasive ventilation could lead to the spread of infection. Because of this, we saw a trend to treat patients in severe respiratory distress with invasive ventilators. Now, we find that many practitioners are using NIV for COVID patients with attention, of course, to infection control precautions. NIV has advantages over invasive ventilation for the patient’s comfort, their ability to eat and talk, for preventing endotracheal-tube-associated infections, and may be appropriate for older patients who may wish to limit invasive care. At six months into the pandemic, we are seeing success with NIV and HFC therapies, particularly when paired with proning (placing a patient on the abdomen to improve matching of blood flow and air in the lungs). Of course, these treatments will continue to evolve as we learn more about the virus and can determine the best ways to treat and mitigate its symptoms. Currently, studies from the early waves of COVID-19 are being published that will offer key insights and learnings to help us best manage the virus in the months to come. Q: What are the top three things you think the medical community needs to consider about the pandemic moving forward? A: First, as physicians we need to learn to be flexible with our use of knowledge under these circumstances. There is a tremendous amount of uncertainty – this virus is novel, and every day we are learning more and more about it. Secondly, we need to learn to expect the unexpected. If there’s anything to take away from this virus, it’s that we can’t be overly confident about our ability to project with data – these are predictions, not facts. Last, we need to use this as a moment in time to explore our capabilities in the medical arena. All around the world, the smartest doctors, scientists, engineers, researchers, and more are working to solve the unprecedented problems brought on by this pandemic. Already, there’s been a tremendous amount of innovation in health care, and I’m excited to see what lasting impacts these will bring. [1] The Philips Respironics E30 Ventilator is provided globally for use under local emergency use authorizations, such as the FDA Emergency Use Authorization for ventilators, Health Canada Interim Order for use in relation to COVID-19, and waiver of CE marking, which authorize its use for the duration of the COVID-19 public health emergency, unless terminated or revoked (after which the products may no longer be used). This device is not FDA cleared or approved.

Share on social media
Topics
Contact

Meredith Amoroso
Philips Global Press Office Tel: +1 724-584-8991
You are about to visit a Philips global content page
Continue