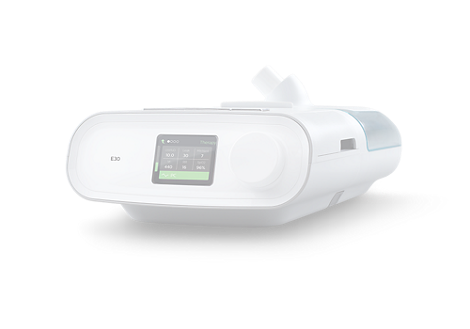
E30
Ventilation Solution
This product is no longer available
Find similar productsAs COVID-19 continues to spread globally, healthcare providers are working diligently to treat soaring numbers of patients at a time when there are too few ventilators to provide care. Philips is responding to this pressing global need by quickly scaling production of the new Philips Respironics E30 ventilator with the needs of healthcare workers and COVID-19 patients.
