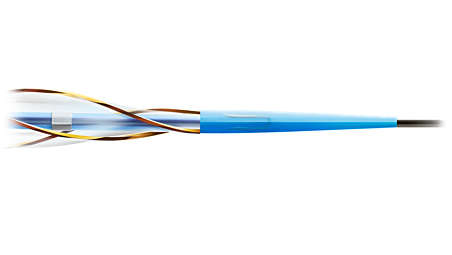
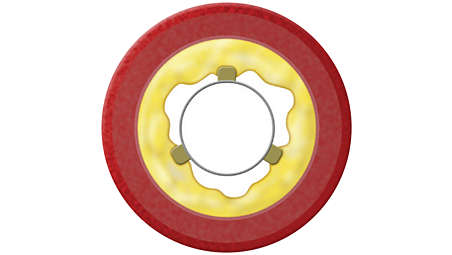
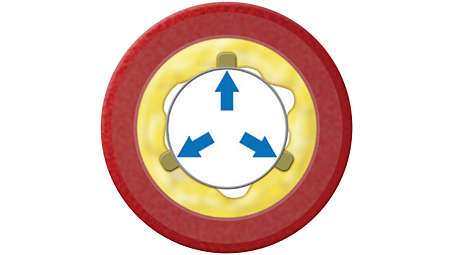
Specifications
- Model number 2200-2006
-
Model number 2200-2006 Balloon diameter - 2 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2010
-
Model number 2200-2010 Balloon diameter - 2 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2015
-
Model number 2200-2015 Balloon diameter - 2 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2506
-
Model number 2200-2506 Balloon diameter - 2.5 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2510
-
Model number 2200-2510 Balloon diameter - 2.5 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2515
-
Model number 2200-2515 Balloon diameter - 2.5 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014in
Guide catheter compatibility - 6F
-
- Model number 2200-3006
-
Model number 2200-3006 Balloon diameter - 3 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3010
-
Model number 2200-3010 Balloon diameter - 3 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3015
-
Model number 2200-3015 Balloon diameter - 3 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3506
-
Model number 2200-3506 Balloon diameter - 3.5 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3510
-
Model number 2200-3510 Balloon diameter - 3.5 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3515
-
Model number 2200-3515 Balloon diameter - 3.5 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2006
-
Model number 2200-2006 Balloon diameter - 2 mm
Balloon length - 6 mm
-
- Model number 2200-2010
-
Model number 2200-2010 Balloon diameter - 2 mm
Balloon length - 10 mm
-
- Model number 2200-2006
-
Model number 2200-2006 Balloon diameter - 2 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2010
-
Model number 2200-2010 Balloon diameter - 2 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2015
-
Model number 2200-2015 Balloon diameter - 2 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2506
-
Model number 2200-2506 Balloon diameter - 2.5 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2510
-
Model number 2200-2510 Balloon diameter - 2.5 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-2515
-
Model number 2200-2515 Balloon diameter - 2.5 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014in
Guide catheter compatibility - 6F
-
- Model number 2200-3006
-
Model number 2200-3006 Balloon diameter - 3 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3010
-
Model number 2200-3010 Balloon diameter - 3 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3015
-
Model number 2200-3015 Balloon diameter - 3 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3506
-
Model number 2200-3506 Balloon diameter - 3.5 mm
Balloon length - 6 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3510
-
Model number 2200-3510 Balloon diameter - 3.5 mm
Balloon length - 10 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- Model number 2200-3515
-
Model number 2200-3515 Balloon diameter - 3.5 mm
Balloon length - 15 mm
Catheter length - 137 cm
Guidewire compatibility - 0.014 in
Guide catheter compatibility - 6F
-
- 1. Mooney M, Teirstein P, Moses J et al. Final results from the U.S. multi-center trial of the AngioSculpt Scoring Balloon Catheter for the treatment of complex coronary artery lesions. Am J Cardiol. 2006;98(8 suppl):121M
- 2. AngioSculpt Test Plan ST-1197 (2008), on file at AngioScore, Inc.
- 3. Fonseca A, Costa JR, Abizaid A, et al. Intravascular ultrasound assessment of the novel AngioSculpt Scoring Balloon Catheter for the treatment of complex coronary lesions. J Invasive Cardiol. 2008;20:21-27.
- AngioSculpt PTCA important safety information
- The AngioSculpt scoring balloon catheter is indicated for use in the treatment of hemodynamically significant coronary artery stenosis, including in-stent restenosis and complex type C lesions, for the purpose of improving myocardial perfusion. *Product availability is subject to country regulatory clearance. Please contact your local sales representative to check the availability in your country.
- The AngioSculpt catheter should not be used for coronary artery lesions unsuitable for treatment by percutaneous revascularization, and coronary artery spasm in the absence of a significant stenosis.
- Possible adverse effects include, but are not limited to: death; heart attack (acute myocardial infarction); total occlusion of the treated artery; coronary artery dissection, perforation, rupture, or injury; pericardial tamponade; no/slow reflow of treated vessel; emergency coronary artery bypass (CABG); emergency percutaneous coronary intervention; CVA/stroke; pseudoaneurysm; restenosis of the dilated vessel; unstable chest pain (angina); thromboembolism or retained device components; irregular heart rhythm (arrhythmias, including life-threatening ventricular arrhythmias); severe low (hypotension)/high (hypertension) blood pressure; coronary artery spasm; hemorrhage or hematoma; need for blood transfusion; surgical repair or vascular access site; creation of a pathway for blood flow between the artery and the vein in the groin (arteriovenous fistula); drug reactions, allergic reactions to x-ray dye (contrast medium); and infection.
- This information is not intended to replace a discussion with your healthcare provider on the benefits and risks of this procedure to you.
- Caution: Federal law restricts the devices referenced on this site to sale by or on the order of a physician.
- Product availability is subject to country regulatory clearance. Please contact your local sales representative to check the availability in your country.
- AngioSculpt RX PTCA is distributed by Biotronik in Australia and New Zealand.