Best-in-class interventional tools built for today’s lab, ready for tomorrow’s innovations.
We envision personalized, efficient and clinically smart cardiac care that helps drive optimal outcomes throughout the patient journey. Our vision offers strategic and focused approach, addressing critical cardiac care needs and meeting better health outcomes, lower cost of care and improved patient and staff experience.
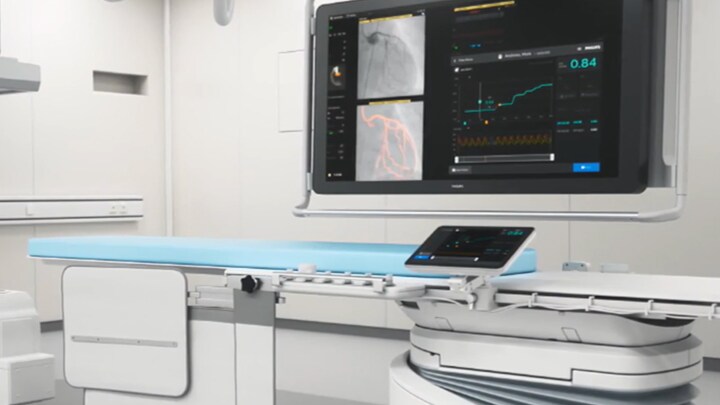
That is why the Philips Interventional Applications Platform — IntraSight — offers a comprehensive suite of clinically proven1-5 imaging, physiology and co-registration6 tools on a modern, secure platform. These best-in-class interventional tools allow you to see clearly beyond the angiogram and ultimately complete your view of the target vessel so you can make fast, informed clinical decisions to treat optimally. IntraSight’s intuitive user interface and simplified workflow contributes to an outstanding user experience in both Philips and non-Philips x-ray labs. IntraSight also helps optimize lab performance with its unique tableside touchscreen module that allows users to run an entire case within the sterile field. Note: IntraSight 7, iFR Co-registration and IVUS Co-registration are not available on IntraSight mobile platform.

Want to learn more about IntraSight?
Stay up-to-date and get informed about interventional cardiology topics. Or get in touch with our sales team.
IntraSight is the only interventional platform that:

Includes iFR physiology
the global gold standard among resting indices with a Class IA recommendation in the ACC/AHA/SCAI and ESC Guidelines.7,8
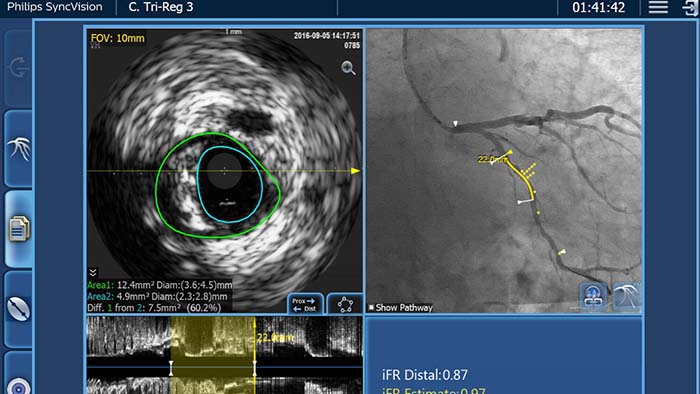
Combines iFR and IVUS data
with the angiogram using iFR Co-registration, IVUS Co-registration and Tri-registration features*
Enables an entire case
to be run tableside without breaking scrub with the touch screen module
Now available, IntraSight on a mobile platform*
IntraSight’s scalable platform can now be experienced on an easy to maneuver mobile cart for all environments, and is ideally suited for acute and non-acute settings. With its customizable platform, you can now select the best-in-class imaging and physiology tools that are right for your coronary or peripheral vascular patients.
*Product availability is subject to country regulatory clearance. Please contact your local sales representative to check availability in your country.
Download the latest brochure here:




IntraSight is smart
Built on a smart, applications-based platform that can scale to meet the evolving needs of your lab when new applications or modalities become available, without the need to purchase new hardware. Only Philips Interventional Applications Platform, IntraSight, offers best-in-class imaging and physiology tools with iFR, iFR Co-registration*, FFR, IVUS, IVUS Co-registration*, and Angio+*. With its modular architecture, IntraSight stays on top of latest advances and important security updates.
Single access point with a shared touch screen module with IntraSight and Azurion.

Only iFR
has clinically validated patient outcome data in the largest physiology studies ever conducted. No other resting index has patient outcome data to support its use.7,8
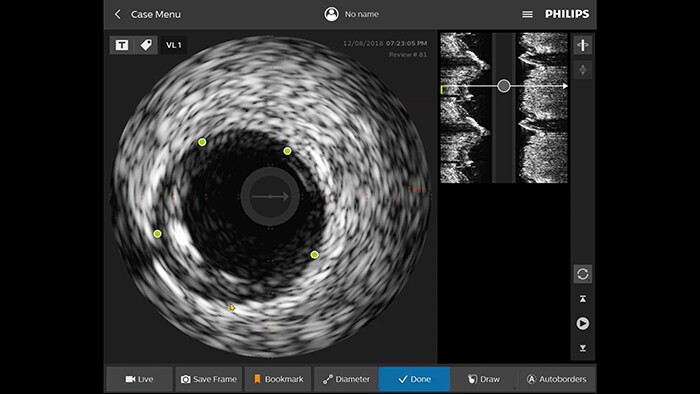
IVUS guidance
is “definitely beneficial” for patients,9 and resulted in a change of treatment plans 74% of the time.10

Secure and safe
First interventional platform to run on Microsoft Windows 10, providing the latest data encryption and advanced protection against cybersecurity threats. Usernames/passwords controlled by administrator and system auto logout safter timeout.
IntraSight is simple
Delivering an outstanding user experience with a modern, intuitive interface that minimizes learning curves and increases workflow confidence.
Easy-to-use because you can:
Video 1
Video 2
IntraSight is seamless
Optimizing lab performance with tableside touchscreen control, systems integration, data management and remote service diagnostics
Increase case efficiency, save time and reduce errors with seamless data
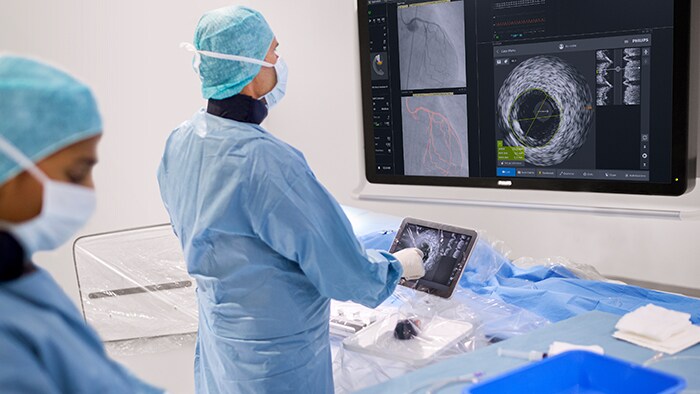
Image 1
Image 2
IntraSight series configurations
The IntraSight platform allows physicians to perform intravascular ultrasound (IVUS) imaging and physiologic measurements of fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) to accurately identify the location of lesions causing ischemia.
IntraSight Mobile is built on the same software as IntraSight and is compatible with the current and future portfolio of Philips IVUS and physiology disposables. It includes a touchscreen panel PC and a ruggedized multi-modality touch screen module mounted on a modern and easy to maneuver cart. Its intuitive user interface and simplified workflow offer clinicians an outstanding user experience while also optimizing lab performance.
-
IntraSight
The IntraSight applications platform is where imaging, physiology, co-registration* and software all come together to clearly identify coronary and peripheral artery disease, and allow for more optimized treatment plans. IntraSight is built on a new foundational platform designed to meet the evolving needs of your lab today and tomorrow.
IGTDINTRSGHT -
IntraSight Mobile
IntraSight Mobile is an easy-to-use, small footprint, digital IVUS imaging and physiology system, designed for peripheral vascular and coronary procedures, operable directly from the sterile field. This is available for the hospital, office-based lab, and ambulatory surgery center environment.
IGTDINTRSGHTMBL
*Philips Mobile Interventional Applications Platform – IntraSight Mobile – is CE marked for sale in Europe and has received FDA 510(k) clearance for sale in the U.S. 1. Davies JE, et al., DEFINE-FLAIR: A Multi- Centre, Prospective, International, Randomized, Blinded Comparison of Clinical Outcomes and Cost Efficiencies of iFR andFFR Decision-Making for Physiological Guided Coronary Revascularization. New England Journal of Medicine, epub March 18, 2017. 2. Gotberg M, et al., iFR-SWEDEHEART Investigators.. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017 May 11;376(19):1813-18233. 3. Patel M. “Cost-effectiveness of instantaneous wave-Free Ratio (iFR) compared with Fractional Flow Reserve (FFR) to guide coronary revascularization decision-making.” Late-breaking Clinical Trial presentation at ACC on March 10, 2018. 4. A. Maehara, M. Matsumura, Z.A. Ali, G.S. Mintz, G.W. Stone. IVUS-guided versus OCT-guided coronary stent implantation. J Am Coll Cardiol Img, 10 (2017), pp. 1487-1503. 5. Choi K, et al. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing ComplexProcedures. JACC: Cardiovascular Interventions. Mar 2019, 4281; DOI: 10.1016/j.jcin.2019.01.227. 6. Co-registration tools available within IntraSight 7 configuration via SyncVision. 7. Lawton J. et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. JACC. 2022;79(2):e21-e129. 8. 2018 ESC/EACTS Guidelines on myocardial revascularization: The task force on myocardial revascularization of the European society of cardiology (ESC) and European association for cardio-thoracic surgery (EACTS). Eur Heart J. 2018;00:1-96. 9. Lotfi, A., Davies, J. E., Fearon, W. F., Grines, C. L., Kern, M. J., & Klein, L. W. (2018). Focused update of expert consensus statement: Use of invasive assessments of coronary physiology and structure: A position statement of the society of cardiac angiography and interventions. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions, 92(2), 336–347. 10. Witzenbichler B, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: The ADAPT-DES study. Circulation. 2014 Jan:129,4;463-470.

Let's get connected
How would you improve interventional procedures? What do you think about the new IntraSight Platform? Are we on the right track? Connect with us via #futureofIGT / IntraSight
#futureofIGT, #futureofCAD, #IntraSight, #CoronaryIntervention, #seeclearlytreatoptimally, #IVUS