Each day at TCT, we will have numerous interactive opportunities for you to learn how you can overcome your
cardiovascular challenges.
Hands-on training
Expand the tabs below to see the highlights, then plan your day.
Join our lunch symposium 12:30 – 1:30 pm
Philips engagement hub
Tuesday, October 24
Advanced image-guided solutions for evolving challenges in SHD interventions
New image-guided solutions continue to evolve, creating workflows that “think” more and more like an imager. Learn more about these workflows – with solutions such as EchoNavigator – and how they continue to drive reproducibility, transparency, and teamwork.

Rebecca Hahn, MD Columbia University Irving Medical Center
Wednesday, October 25
Navigating the future of contemporary PCI: Physician-to-physician education and cutting-edge technology
Discover training techniques and treatment strategies to improve overall procedural outcomes, open the door for patients once not considered candidates for PCI, and streamline workflows with integrated tools.

Karim Al-Azizi, MD Baylor, Scott & White Health – The Heart Hospital

Evan Shlofmitz, DO St. Francis Hospital
Thursday, October 26
Why 3D ICE should be a part of all SH programs – our experience adopting and expanding 3D ICE into multiple procedure types
Hear how we have learned to maximize the benefits of 3D ICE in mitral, tricuspid, LAAO, and more. Questions and discussion encouraged.

UC Davis Health

UC Davis Health
Heart and Vascular Services
A powerful ecosystem for cardiovascular care
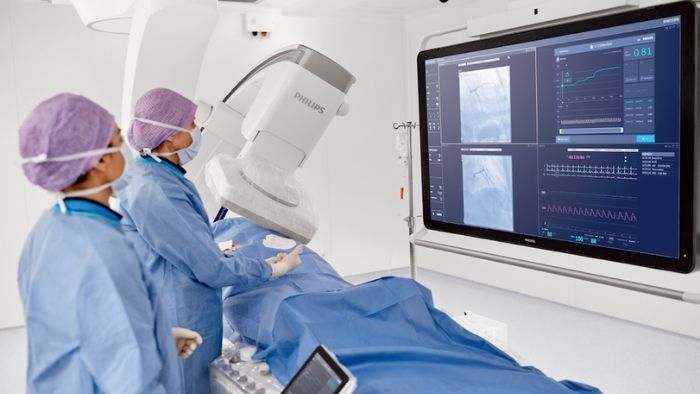
Coronary artery disease (CAD)
Unlock the combination of quick, confident diagnosis and efficient, effective treatment for CAD.
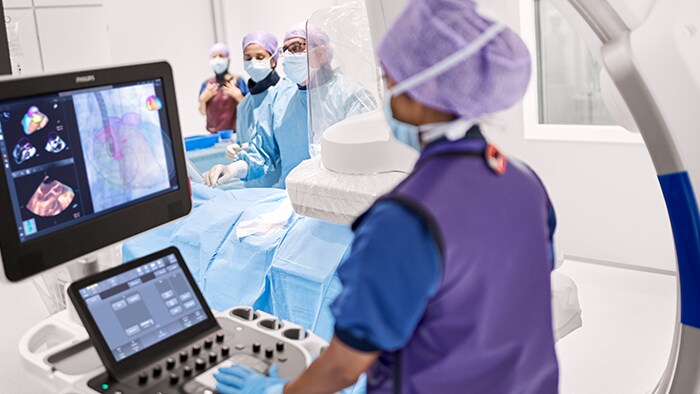
Structural heart disease (SHD)
Improve SDH care and reduce procedure time through exceptional image guidance and seamless collaboration.
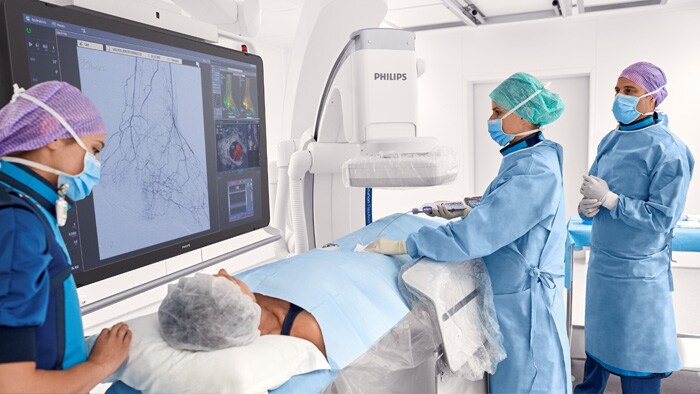
Peripheral vascular disease (PVD)
Redefined outcomes for our vascular patients with innovations that simplify therapy and help reduce variability.
1. Elgendy IY et al. Outcomes with intravascular ultrasound-guided stent implantation: a meta-analysis of randomized trials in the era of drug eluting stents. Circ-CardiovascInterv.2016;9:e003700 2. Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patientsenrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338-1347. Hyperlink http://www.ajconline.org/article/S0002-9149(14)00549-9/abstract 3. Witzenbichler B, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: The ADAPT-DES study. Circulation. 2014 Jan:129,4;463-470. 4. Singh V, Badheka AO, Arora S, et al. Comparison of in-hospital mortality, length of hospitalization, costs, and vascular complications of percutaneous coronary interventions guided byultrasound versus guided by angiography. Am J Cardiol. Online 18 Feb 2015. 5. Escaned, et al. The DCR4Contrast Trial. Presented at EuroPCR as Late-Breaking clinical data May 2023.

