Philips’ new advanced imaging ultrasound system provides a cost-effective solution to clinicians in a wide range of care settings
May 6, 2013
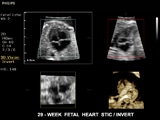
New Orleans, USA – Royal Philips Electronics (NYSE: PHG, AEX: PHIA) today showcased its latest addition to the ClearVue ultrasound family of products at the American College of Obstetricians and Gynecologists 61st Annual Clinical Meeting in New Orleans, Louisiana, May 4-8, 2013. The ClearVue 650 is a lightweight and cost-effective system that spans a range of applications from obstetrics and gynecology to cardiology, abdominal, vascular, breast, musculoskeletal, urology and general imaging. Philips new ultrasound system includes cutting-edge tools such as four dimensional (4D) imaging technology called Spatio Temporal Image Correlation (STIC) that allows clinicians to quickly inform patients about any rare or complex heart abnormalities in the fetus. It produces clear, easy-to-read images that can help clinicians accurately determine gestation age, date of conception and other important information about the developing fetus, including visual confirmation of the pregnancy, a reliable image of the fetus’ well-being and heart-health, and embryonic movement a full two weeks earlier in gestation than many existing ultrasound systems. Additionally, Philips new ultrasound system uses pulsed wave Tissue Doppler Imaging (TDI) technology that provides velocity mapping of cardiac tissue to evaluate cardiac function and wall motion. It also features Auto Face Reveal, a technology that sculpts away overlaying tissue to clearly reveal the face of the unborn baby. A published study suggests that Auto Face Reveal, and the technology powering it, may even enhance parental-fetal bonding. “The Philips ClearVue 650 is more evidence of Philips’ continuing commitment to innovate and help clinicians improve patient outcomes and the healthcare delivery experience,” said Conrad Smits, General Manager, Ultrasound, Philips Healthcare. “It is a system that will better serve the health community with its excellent image quality and simplified workflow for clinicians across a broad range of specialties including obstetrics, gynecology and cardiology.” Additional features and benefits available in Philips ClearVue 650 include: The new Philips ClearVue 650 ultrasound system uses 3D/4D technology to enhance clinical insight and diagnostic confidence. The 3D imaging technology creates depth, whereas the 4D technology adds an element of movement. The ClearVue 650 is the most recent addition to the ClearVue family of Philips Ultrasound products, including the 350 and 550. All systems feature the proprietary Active Array technology, which enhances maneuverability and enables superb image quality. Visit Philips at ACOG 2013 at booth #1801 to learn more or speak with a Philips representative. For more information, please go to www.philips.com/clearvue650. 1 Pretorius D, Gattu S, Ji E, Hollenbach K, Newton R, Hull A, et al. Prexamination and postexamination assessment of parental-fetal bonding in patients undergoing 3-/4-dimensional obstetric ultrasonography. J Ultrasound Med. 2006;25:1411–1421.New Auto Face Reveal facilitates visualization of the baby’s face, possibly enhancing parental-fetal bonding.1
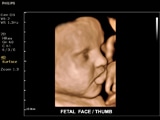
ClearVue 650 "Fetal face"