June 05, 2018
Philips receives U.S. FDA 510(k) clearance for its Ingenia Elition MR solution
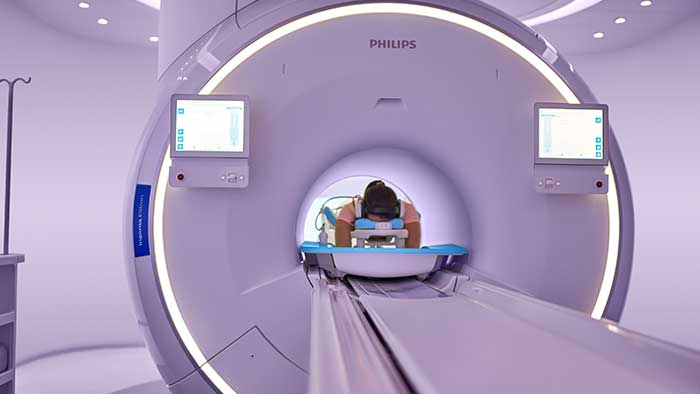
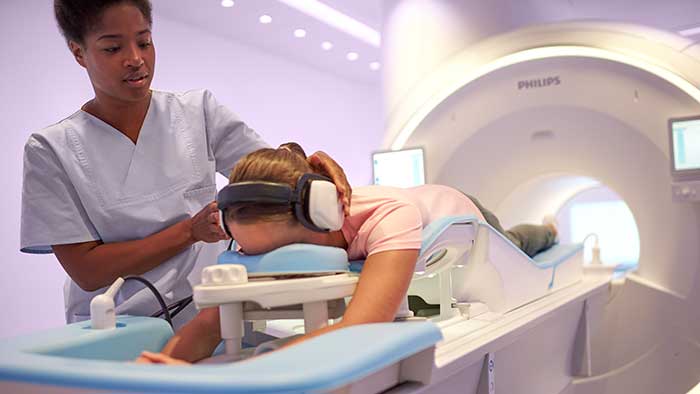
With first U.S. commercial installation at Hennepin Healthcare, the Philips Ingenia Elition performs MRI exam times up to 50% [1] faster with no compromise in image quality
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Ingenia Elition 3.0T MR solution and two clinical applications, Philips Compressed SENSE and 3D APT. This integrated suite of innovations enables clinicians to perform exams up to 50% faster [1], increase diagnostic confidence and improve the patient experience. The first commercial installation of the Philips Ingenia Elition in the U.S. has recently been completed at Hennepin Healthcare, a comprehensive healthcare system in Minneapolis. “Together, the Ingenia Elition and our new clinical applications make producing high-quality images fast and easy, enabling prompt diagnosis and setting the stage for effective treatment,” said Arjen Radder, Global Business Leader for MR at Philips. “We’re receiving a strong positive reaction from our customers as we continue to roll out our all-new Ingenia digital MR portfolio. It’s providing healthcare organizations like Hennepin Health with innovative solutions that seamlessly connect data, technology and people to drive the highest quality of care.” In today’s healthcare landscape – where reimbursements are becoming value-based and chronic conditions lead to more MR procedures and longer waiting times – there is an ever-increasing pressure on the radiology department. Philips is responding to these challenges through the development of new systems and clinical applications that improve image quality and the patient and staff experience, as well as operational efficiency. “To deliver fast, consistent and accurate diagnoses, our staff need to be supported with technology that gives them the ability to provide the best patient care, in an efficient and cost-effective way,” said Dr. Chip Truwit, MD, Chair, Radiology, Hennepin Healthcare. “Philips’ Ingenia Elition plays a critical role in elevating the standard of care for our patients in imaging and in improving overall operations in our new imaging center.” Improving diagnostic confidence and the patient experience Philips Ingenia Elition and the entire digital MR portfolio empower a faster, smarter and simpler path to diagnosis – providing patients and radiologists with the following benefits: All-new Ingenia digital MR portfolio The Ingenia Elition is part of Philips’ all-new Ingenia digital MR portfolio, which supports radiology departments to enhance productivity, improving the patient and staff experience, while delivering better value-based care through improved patient outcomes at lower costs. In addition to receiving U.S. FDA approval the Ingenia Elition 3.0T is also available for sale in Europe. [1] Using Compressed SENSE technology and compared to Philips exams without Compressed SENSE [2] SmartExam is not available to patients with MR Conditional implants [3] Togao et al. (2014) Neuro-Oncology [4] Park KJ et al. (2016) European Radiology [5] Results from case studies are not predictive of results in other cases. Results in other cases may vary [6] Compared to the average of the other five Philips MR scanners without Ambient Experience and In-bore Connect. Results from case studies are not predictive of results in other studies. Results in other cases may vary [7] Based on in-house testing
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips' health technology portfolio generated 2017 sales of EUR 17.8 billion and employs approximately 74,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
For further information, please contact:

Mark Groves
Philips Global Press Office Tel.: +31 631 639 916
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue