Feb 11, 2020
Philips announces DEFINE GPS global multicenter study to assess outcomes of PCI procedures guided by integrated iFR and interventional X-ray images
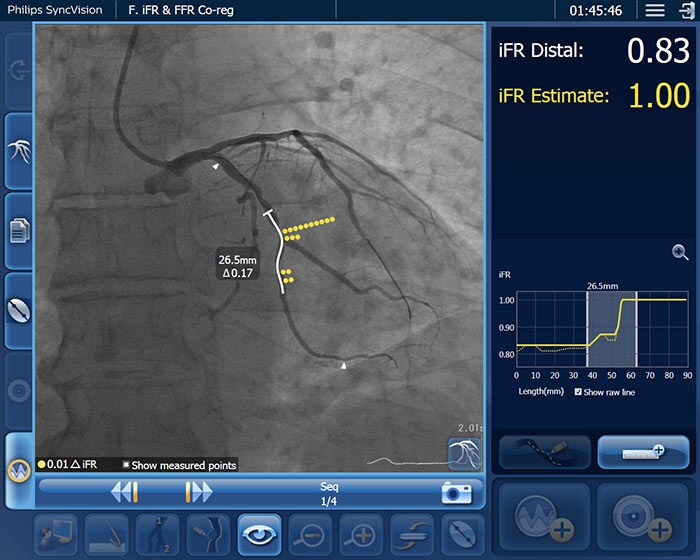
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced a new randomized controlled trial to assess patient outcomes after receiving a percutaneous coronary intervention (PCI) guided by Philips’ unique co-registration platform, which combines data of an instant wave-Free Ratio (iFR) measurement and the angiogram (interventional X-ray image), compared with today’s standard of care treatment guided by an angiogram alone. PCI is one of the main treatment options to open narrowed coronary arteries of the heart in patients with coronary artery disease. European and US guidelines already endorse the use of coronary physiologic measurements with iFR and Fractional Flow Reserve (FFR) for diagnostic purposes to determine the significance of a narrowed coronary artery. The DEFINE GPS (Distal Evaluation of Functional performance with Intravascular sensors to assess the Narrowing Effect: Guided Physiologic Stenting) study will be the first time that the use of iFR in conjunction with the Philips Image-Guided Co-registration System (SyncVision) is evaluated for PCI guidance and the optimization of treatment outcomes. PCI is an image-guided, minimally invasive treatment to open a coronary artery blockage (stenosis) that is causing a reduced blood flow (ischemia) to heart tissue. Under the current standard of care, clinicians navigate a balloon catheter and coronary stent to the treatment area using interventional X-ray guidance (coronary angiography). In the study, an iFR pullback measurement, which uses pressure wires to map the pressure profile of the disease distribution along the length of the affected vessel, will be overlaid on the X-ray image to guide treatment. iFR is a next-generation physiologic measurement that uses the same pressure guide wires and equipment as FFR but avoids the administration of hyperemic agents to patients.
With the DEFINE PCI study we observed that the current approach to PCI has limitations for identifying the locations of physiologically significant arterial lesions. With DEFINE GPS we will be able to determine if a physiology-based PCI approach results in superior patient outcomes when compared with standard angioplasty.
Allen Jeremias
MD of St. Francis Hospital, Roslyn, NY, and Cardiovascular Research Foundation, New York, NY
“PCI has made a major positive impact on many coronary artery disease patients’ lives,” said Allen Jeremias, MD of St. Francis Hospital, Roslyn, NY, and Cardiovascular Research Foundation, New York, NY, U.S. and principal investigator of the DEFINE GPS study. “However, when we look back at all the major, high-quality stent trials over the past 20 years we see that around 20-30% of patients continue to have recurring chest pain at one year after receiving treatment. With the DEFINE PCI study we observed that the current approach to PCI has limitations for identifying the locations of physiologically significant arterial lesions. With DEFINE GPS we will be able to determine if a physiology-based PCI approach results in superior patient outcomes when compared with standard angioplasty.” “As coronary stenting is applied to increasingly complex patients, it is essential that we ensure that all segments of coronary artery disease that need treatment are treated, a process that we believe can be facilitated by iFR assessment of the entire coronary artery, co-registered to the angiogram.” said Dr. Gregg W. Stone, chairman of the DEFINE GPS trial, Director of Academic Affairs for the for the Mount Sinai Heart Health System, Professor of Medicine (Cardiology) and Professor of Population Health Sciences and Policy at the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY. “We are thrilled to be able to examine the extent to which this technique improves patient outcomes in the large-scale DEFINE GPS trial.” The global multicentre, prospective, randomized controlled DEFINE GPS study will investigate the impact of iFR Co-registration on both outcomes and cost effectiveness. The primary endpoint is target vessel failure (a composite of cardiac death, target vessel Myocardial Infarction and ischemia-driven target vessel revascularization) or rehospitalization for progressive or unstable ischemia at two years. “iFR continues to be adopted into clinical practice, with mounting evidence that this innovative technology contributes to improving outcomes, reducing costs [1, 2, 3] and enhancing the patient experience,” said Chris Landon, Senior Vice President and General Manager Image Guided Therapy Devices, Philips. “This major study will provide a definitive answer to the question of the overall improvement resulting from the use of a functional guidance strategy on patient outcomes and cost.” The Philips Image-Guided Co-registration System (SyncVision) is part of Philips’ unique portfolio of systems, smart devices, software and services in image-guided therapy, which combine to provide healthcare providers with sophisticated, procedure-oriented solutions. The DEFINE GPS study is sponsored by Philips with the Cardiovascular Research Foundation overseeing core lab and clinical event committee activities. The first patients will be recruited in the second half of 2020. [1] Davies JE, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017 May 11;376(19):1824-1834. [2] Gotberg M, et al. iFR Swedeheart Investigators. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017 May 11;376(19):1813-1823. [3] Tonino, et al. Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention. N Engl J Med. 2009;360(3):213-224.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 80,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Mark Groves
Philips Global Press Office Tel.: +31 631 639 916
You are about to visit a Philips global content page
Continue
Fabienne van der Feer
Philips Image Guided Therapy Tel: + 31 622 698 001
You are about to visit a Philips global content page
Continue