Mar 15, 2022
Latest release of Philips Capsule Surveillance receives FDA clearance
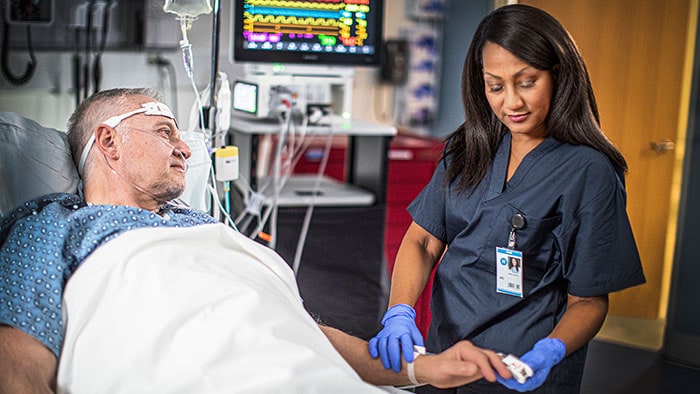
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, announced today at HIMSS22 that the latest Philips Capsule Surveillance solution has received 510(k) market clearance from the U.S. Food & Drug Administration (FDA), paving the way for widespread deployment across healthcare systems in the USA. Capable of utilizing streaming data from virtually any connected medical device, the solution aggregates patient data, analyses it to generate actionable insights and alerts, and sends timely notifications to the patient’s caregivers so that they can intervene before deterioration progresses further. This latest release of Philips Capsule Surveillance includes expanded interoperability into hospitals’ existing mobile clinical communication and collaboration tools and electronic intensive care units (eICUs) and virtual care population health management systems, offering more visibility on live streaming data, waveforms, device alarms and contextual alerts.
Properly implemented clinical surveillance has the potential to significantly improve patient outcomes by helping to avoid deterioration, while also improving the care team experience via clinical decision support and minimizing the burden of false and clinically unactionable alarms.
Elad Benjamin
General manager of Clinical Data Services at Philips
“This FDA clearance of the latest release of clinical surveillance solution enables more integrated viewing options within EMR and HIT tools through the secure web-based user interface. The updated intended use provides flexible deployment configurations that Philips Capsule can offer to hospitals and health systems in the USA,” said Elad Benjamin, general manager of Clinical Data Services at Philips. “Properly implemented clinical surveillance has the potential to significantly improve patient outcomes by helping to avoid deterioration, while also improving the care team experience via clinical decision support and minimizing the burden of false and clinically unactionable alarms.”
Enhanced data visibility at eICU and virtual care locations
Philips Capsule Surveillance offers an enterprise-wide solution that complements eICU telehealth command center solutions, such as Philips eCareManager, by bringing together live-streaming patient data across multiple acuity settings, equipment brands and device types to show each patient’s immediate status.
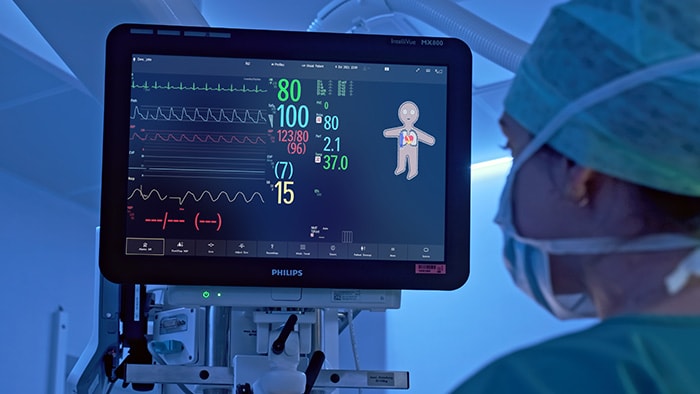
Smart rule clinical decision support
Built on the vendor-neutral Philips Capsule Medical Device Information Platform, which captures and normalizes streaming data from a network of connected devices, the Philips Capsule Surveillance software continuously analyzes patient data using patented technology to identify deteriorating conditions and critical events. The software applies a set of smart rules based on clinical parameters and current best-practice guidelines that can be tailored to an individual hospital’s protocols for specific morbidities. Philips Capsule Surveillance allows clinicians to see patient data and patient monitor settings and alarms from multiple device types without needing to enter the patient’s room. When caring for infectious patients, this can help reduce the risk to clinical staff. Early identification of deteriorating patient conditions can also help to avoid complications and escalations, contributing to lower cost of care, while the ability to remotely monitor large numbers of patients and focus resources where needed can help mitigate the shortage of experienced clinicians. The latest version of Philips Capsule Surveillance will be released in Q2 to limited sites in the USA. For more information, visit https://capsuletech.com/capsule-clinical-surveillance. For more information on Philips’ full portfolio of connected care solutions being showcased in booth #2501 at the HIMSS22 Global Health Conference & Exhibition, please visit www.philips.com/himss and follow @PhilipsLiveFrom for #HIMSS22 updates throughout the event.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2021 sales of EUR 17.2 billion and employs approximately 78,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.