Jul 18, 2023
First patient in the USA enrolled in global WE-TRUST trial to accelerate stroke diagnosis and treatment marks milestone in global reach
Enrollment at Baptist Stroke & Cerebrovascular Center in Jacksonville, Florida, is the first in the USA for the WE-TRUST clinical trial, evaluating the potential benefits of a Direct to Angio Suite treatment pathway for stroke patients
Cambridge, MA – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced enrollment of the first patient in the USA into the large-scale WE-TRUST* multicenter randomized controlled clinical trial to assess whether a Direct to Angio Suite (DTAS) workflow improves outcomes for early time-window stroke patients compared to the conventional MR/CT scan before transfer to the Angio suite pathway. Recruited at the Baptist Stroke & Cerebrovascular Center, a comprehensive stroke center in Jacksonville (Florida, USA), this step represents an important international expansion of the WE-TRUST trial, which is already running in several European and South American countries including Spain, Turkey, Germany, Brazil, The Netherlands, Argentina and France.
By incorporating US patients into the WE-TRUST trial, we aim to help provide data and outcomes that will inform clinical workflows, that in turn (and more importantly), benefit patients across the globe.
Ricardo Hanel, MD, PhD
Neurosurgeon, Director of the Baptist Neurological Institute
“Every second counts for acute stroke patients. It is critically important to act quickly and treat them as soon as possible for better results and a better quality of life,” said Ricardo Hanel, MD, PhD, neurosurgeon, Director of the Baptist Neurological Institute. “By incorporating US patients into the WE-TRUST trial, we aim to help provide data and outcomes that will inform clinical workflows, that in turn (and more importantly), benefit patients across the globe.”
Globally, one in four people over the age of 25 will suffer a stroke during their lifetime, currently resulting in over 12 million strokes each year [1]. The World Stroke Organization has estimated that six and a half million people a year died from stroke in 2019 [1], making it the second leading cause of death worldwide, while the U.S. National Stroke Association estimates that 40% of those who survive experience moderate to severe impairments that require special needs for the rest of their lives [2]. The key to reducing this excessive burden of death and disability is diagnosing and treating stroke patients within a few hours of an attack.
WE-TRUST
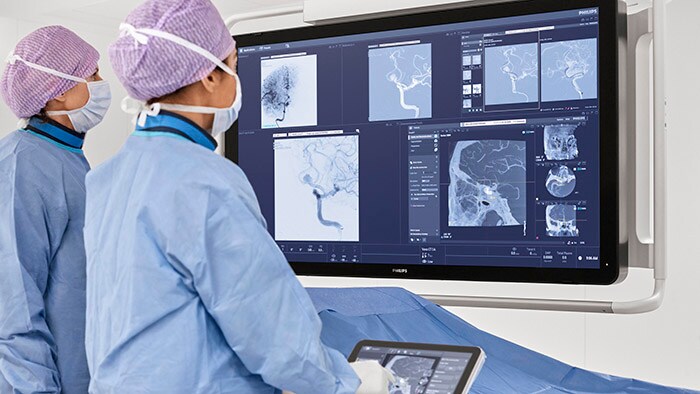
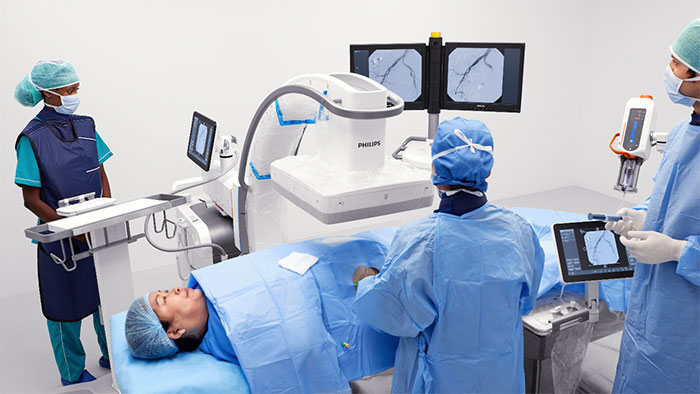
The WE-TRUST (Workflow Optimization to Reduce Time to Endovascular Reperfusion for Ultra-fast Stroke Treatment) trial investigates the clinical impact of the DTAS approach, which combines stroke diagnosis and treatment in a single angio suite session. Enabled by a cone-beam CT (CBCT) imaging tool already integrated into interventional angio suite systems such as Philips Image Guided Therapy System – Azurion, the DTAS approach can potentially reduce the time to treatment for early time-window stroke patients (less than six hours after stroke onset). Every 30 minutes of delay before treatment reduces the chance of a good outcome by 14% [1], and every hour ages the brain by 3.6 years compared to a normally aging brain [2].
“At Philips, we are convinced that direct-to-angio workflows, in which stroke patients are diagnosed and treated in the same room, has the potential to save precious minutes and even more precious brain function,” said Atul Gupta, Chief Medical Officer for Image Guided Therapy at Philips. “While that belief is already supported by the results of several single-center studies, we need a clinical trial with the scale and reach of WE-TRUST to determine whether direct-to-angio has the potential to become the gold standard for stroke care.”
WE-TRUST is a multicenter, prospective, randomized controlled, open-label, blinded-endpoint trial that aims to engage 16 leading strokes sites and enroll a total of more than 500 patients across the United States, Brazil, Argentina, the Netherlands, France, Germany, Spain and Turkey. More information about the study can be found at wetrust-study.com.
Recently, Philips announced that the results of a health economics analysis recently published in the Journal of NeuroInterventional Surgery (JNIS) show that Philips’ direct-to-angio treatment pathway for stroke patients can save more than USD 3,000 per patient.
*The WE-TRUST (Workflow Optimization to Reduce Time to Endovascular Reperfusion for Ultra-fast Stroke Treatment) clinical trial is sponsored by Philips
[1] World Stroke Organization (WSO): Global Stroke Fact Sheet 2022 (https://www.world-stroke.org/assets/downloads/WSO_Global_Stroke_Fact_Sheet.pdf)
[2] U.S. National Stroke Association
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and well-being through meaningful innovation. Philips’ patient- and people-centric innovation leverages advanced technology and deep clinical and consumer insights to deliver personal health solutions for consumers and professional health solutions for healthcare providers and their patients in the hospital and the home. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, ultrasound, image-guided therapy, monitoring and enterprise informatics, as well as in personal health. Philips generated 2022 sales of EUR 17.8 billion and employs approximately 74,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Topics
Contacts

Avi Dines
Philips North America Tel: +1 781-690-3814
You are now exiting the Philips United States (US) site and entering the Philips global site. This content is intended for a global audience. It may not apply to the US and should not be interpreted as meeting US standards, executive orders or regulations.
ContinueMedia assets