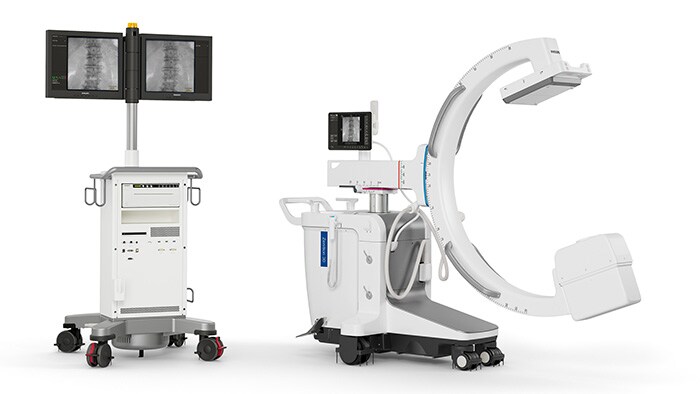
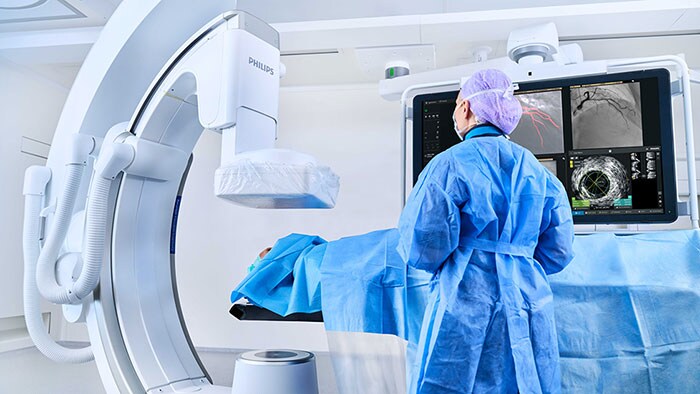
Philips Zenition 30 mobile C-arm receives FDA 510(k) clearance, helping surgeons deliver image-guided therapy to more patients at lower cost
Apr 22, 2024 | 4 minute read
Cambridge, MA, USA – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced its Philips Zenition 30 mobile C-arm has received FDA 510(k) clearance, making image-guided surgical procedures available to more US patients at lower cost.
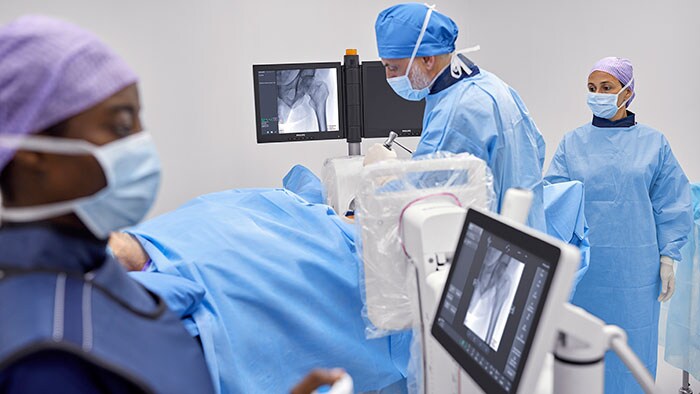
By giving surgeons greater flexibility, control, and personalization of C-arm movement and user settings, Zenition 30 reduces their dependency on support personnel, helping alleviate staff shortages that limit patient access and increase waiting times. By reducing the number of support staff required during procedures, the Zenition 30 also addresses the budgetary constraints that plague many of today’s hospital systems. The enhanced workflow efficiency offered by intuitive C-arm control from alongside the patient table means surgeons can treat more patients yet also spend more time focusing on each patient, leading to a better patient and staff experience.
Based on our Zenition platform’s proven ease of use and workflow efficiency, the new Zenition 30 offers a unique combination of personalized control and image clarity to enhance the speed and accuracy of decision making for a range of clinical procedures at a price point that meets today’s economic and business goals.
“Based on our Zenition platform’s proven ease of use and workflow efficiency, the new Zenition 30 offers a unique combination of personalized control and image clarity to enhance the speed and accuracy of decision making for a range of clinical procedures at a price point that meets today’s economic and business goals,” said Mark Stoffels, General Manager Philips Image Guided Therapy Systems.
High quality imaging
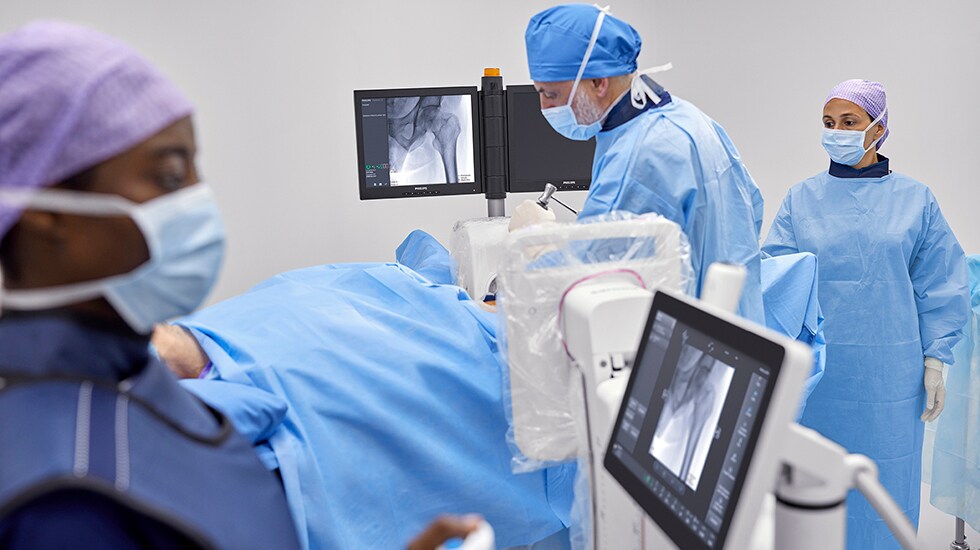
Superior image quality is critical to patient outcomes during image-guided procedures, but to ensure the safety of patients and staff it should not involve excessive X-ray exposure. Featuring Philips’ latest-generation flat detector technology, advanced imaging algorithms, and personalized user profiles, Zenition 30 delivers superior image quality coupled with dose efficiency. It also features automatic workflow customization, automatically adjusting to an individual surgeon’s preferred settings and way of working from the moment they log on. Fewer manual adjustments contribute to smoother procedures and more first-time-right imaging. Philips BodySmart software further enhances a surgeon’s ability to capture consistent images without the need for manual adjustments when the target anatomy is off-center. Via the optional table-side touch screen module, surgeons can also select from a range of application-specific protocols for different types of surgery.
For patients with metal implants, Philips MetalSmart software automatically adjusts image contrast and brightness to improve image quality and eliminate blooming while maintaining low X-ray dose. A dedicated pediatric mode automatically reduces dose rates for young patients. The wide range of surgical procedures facilitated by the Zenition 30 is further enhanced by the system’s digital subtraction angiography (DSA) capability, allowing clear imaging of vasculatures.
Greater control
In addition to rapid set-up and protocol selection, the Zenition 30 also offers surgeons much greater control over C-arm positioning during surgical procedures, with easily reachable pushbuttons on the flat detector allowing them to unlock and adjust the arm’s horizontal, orbital, and rotational positioning from within the sterile field. An electromagnetic braking system is provided on either side of C-arm stand that eliminates the manual effort required for technicians to release and apply conventional mechanical brakes.
In independent usability studies involving clinicians around the world who were offered hands-on experience of the Zenition 30 in simulated environments, 95% said they believed its enhanced Surgeon Control would allow them to work independently [1], while 84% believed that Zenition 30’s personalized image quality profiles meant fewer images might be needed during a procedure because the first image already incorporates their preferred settings [2].
The Zenition mobile C-arm platform brings together innovations in image capture, image processing, ease-of-use, and versatility pioneered on Philips’ highly successful Windows® based Zenition platform.
Sources [1] Results obtained during claims substantiation study performed in February and September 2022 by Use-Lab GmbH, an independent company. Response is based on 50 respondents ( 26 surgeons & radiologists and 24 technologists & nurses) from the EU and US, who answered a questionnaire subsequent to a usability study with additional hands-on time with the system.
[2] Compared to its predecessor BV Endura 2.3.
Media contacts
You are now exiting the Philips United States (US) site and entering the Philips global site. This content is intended for a global audience. It may not apply to the US and should not be interpreted as meeting US standards, executive orders or regulations.
Continue