Jul 29, 2020
Philips launches pre-hospital wireless monitoring solution for emergency medical response in U.S.
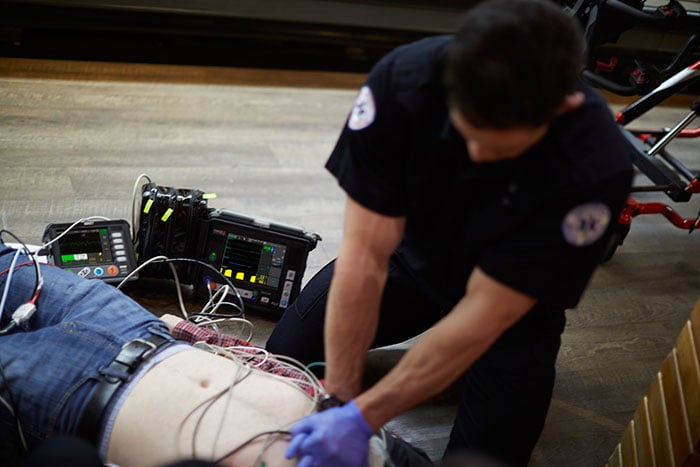
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of its remote monitoring and defibrillator solution (Tempus ALS) for pre-hospital settings in the U.S. The solution is a complete end-to-end system that combines innovative hardware and advanced software to expand the pre-hospital scope of care for first responders. The professional defibrillator (Tempus LS-Manual) is the final element of the overall solution to receive 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is now available for sale in the U.S. market. As a unique modular platform, the remote monitoring and defibrillator solution (Tempus ALS) consists of a remote portable vital signs patient monitor (Tempus Pro), and remote professional defibrillator (Tempus LS-Manual). While the monitor and defibrillator can be used separately, the devices also connect wirelessly to share data and transfer vitals, waveforms and images into Philips web-based software platform (IntelliSpace Corsium). The software platform provides robust, real-time transfer of clinical data and events, interactive ECG measurement, two-way communication and more, enabling rapid clinical and transport decision support and seamless electronic patient care recording (ePCR) integration outside the hospital in emergency settings.
In emergency situations, where seconds count, having access to advanced patient data collection and sharing and real-time secure data streaming, can help inform confident treatment and transport decisions outside the hospital.
Arman Voskerchyan
General Manager of Therapeutic Care at Philips
“In emergency situations, where seconds count, having access to advanced patient data collection and sharing and real-time secure data streaming, can help inform confident treatment and transport decisions outside the hospital,” said Arman Voskerchyan, General Manager of Therapeutic Care at Philips. “The integrated remote monitoring and defibrillator solution combined with our web-based software platform will help front line responders provide emergency care, diagnosis and treatment - including defibrillation therapy, data management and clinical and operational efficiency features – in a fully integrated solution.” Emergencies and care events outside the hospital continue to rise, with an estimated 240 million calls made to 9-1-1 in the U.S. each year [1]. In addition to the stress of the unknown and what to expect at the scene of the call, emergency medical providers must deal with manual handling issues. Equipment carried is heavy, often damaged due to use in unpredictable conditions and has limited data connectivity – inhibiting the ability for on-scene support. In an effort to address these challenges, both elements of the Philips remote monitoring and defibrillator (Tempus ALS) solution are designed with a small, rugged exterior and long-lasting battery to allow emergency medical providers to focus on caring for the patient without the hassle or distraction of bulky equipment.
Philips leadership in Emergency Care solutions
Earlier this year, Philips launched its new emergency care informatics suite in the U.S. market, previously in use in Europe, helping care teams spot life-threatening conditions remotely, improve accuracy of support from on-scene crews, and enhance tailoring of in-hospital care based on pre-hospital physiology. In October 2019, Philips announced a first-of-its-kind collaboration with Air Ambulance Kent Surrey Sussex (AAKSS) where helicopter Emergency Service (HEMS) teams were able to live stream patient medical information from the scene to the hospital through the Philips pre-hospital solution. Philips offers a wide range of emergency care offerings, including automated external defibrillators (AEDs), advanced life support monitors, and more. Visit Philips Emergency Care and Resuscitation for more information on the remote monitoring and defibrillator solution and Philips broad portfolio of Emergency Care solutions.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.