Jun 12, 2020
Philips launches obstetrics monitoring solution to support clinicians and expectant mothers during COVID-19 pandemic
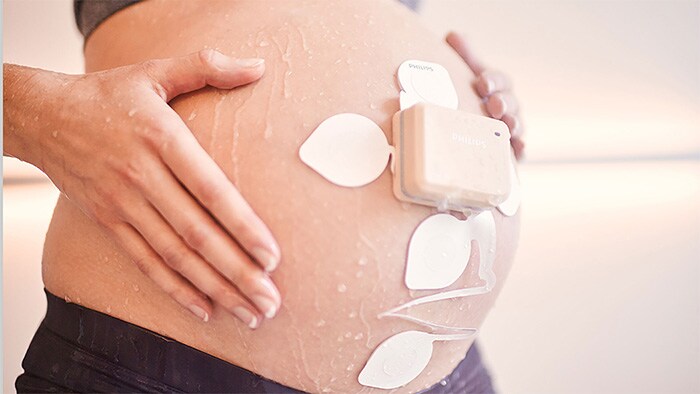
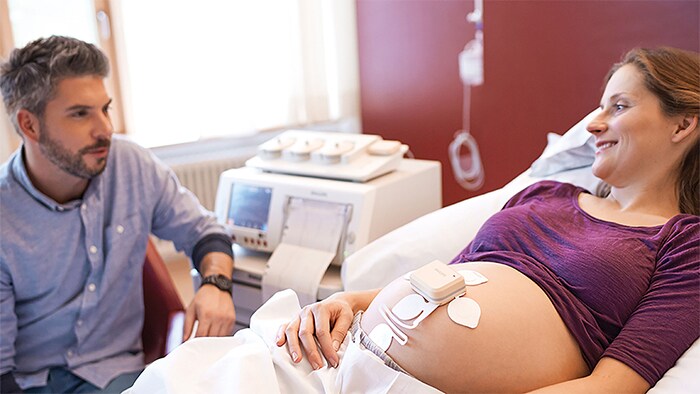
Amsterdam, The Netherlands - Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced an addition to its remote patient monitoring suite supporting at-risk populations during the COVID-19 emergency. The new wireless Avalon CL Fetal and Maternal Pod and Patch aim to reduce unnecessary physical interactions between clinicians and patients, which is of particular importance during the COVID-19 pandemic. The patch is part of a broader innovative high-risk pregnancy solution which includes Philips perinatal analytics, and visualization software as well as an ultra-portable battery-operated fetal monitor. Up to half a million women may deliver their babies while infected with COVID-19 in 2020. Additionally, pregnant women who have not been diagnosed with COVID-19 are interested in ways they can minimize their time in a hospital to limit their exposure to the disease. While clinicians are treating COVID-19 patients in isolation rooms, and accommodating home visits and births when possible, properly managing patient care while reducing the risk of exposure for care providers requires tools that enable remote monitoring of vitals. The Fetal and Maternal Pod and Patch allow for continuous, non-invasive monitoring of maternal heart rate, fetal heart rate, and uterine activity with a single-use, 48-hour, disposable electrode patch placed on the mother’s abdomen. The patch is designed to be placed on the patient by a clinician only once, unlike traditional elastic belts and sensors that require frequent repositioning.
“Remote monitoring during labor has always provided multiple benefits to expectant mothers, including comfort, mobility, and flexibility. But during the COVID-19 pandemic, the need for mobile solutions during pregnancy is greater than ever,” said Peter Ziese, General Manager, Monitoring & Analytics at Philips. “Philips has been dedicated to providing the best quality care for expectant mothers for more than fifty years. This new solution builds on our commitment to provide integrated continuous monitoring capabilities for high risk pregnancies. With this new patch, clinicians now have access to an innovative tool to help monitor pregnant women during COVID-19, helping to deliver comfort to these mothers during a particularly stressful time.” “The fact that the new sensors are disposable and don’t require constant repositioning has been particularly useful for us in the peak of the COVID-19 wave in March and April in Lisbon,” said Prof. Dr. Diogo Ayres-de-Campos, University of Lisbon, and President-Elect of the European Association of Perinatal Medicine (EAPM).
With this new patch, clinicians now have access to an innovative tool to help monitor pregnant women during COVID-19, helping to deliver comfort to these mothers during a particularly stressful time.
Pieter Ziese
General Manager, Monitoring & Analytics at Philips
The Philips OB solution consists of the Avalon Fetal monitoring portfolio, featuring Philips proprietary Smart Pulse technology, and an obstetrical information management system for continuous data flow to the EMR, which includes:
The Avalon Fetal and Maternal Pod and Patch received CE Mark clearance in 2019 and is available in many EU countries [2], as well as Australia, New Zealand and Singapore. Philips has a comprehensive portfolio of services and solutions which can help to support the delivery of high-quality care to COVID-19 patients. It includes secure, connected and intelligent approaches to diagnosis, treatment and predictive monitoring in the hospital, plus screening, remote patient monitoring and care at home. With healthcare under more pressure than ever before, Philips’ telehealth and AI-enabled data analytics can help support workflows, facilitate remote collaboration and optimize resources. Philips’ COVID-19-related solutions are designed for rapid deployment and scalability. For more information on how Philips is addressing COVID-19 globally, please visit the Philips centralized COVID-19 hub. [1] https://www.fda.gov/media/137286/download. [2] The Avalon Fetal and Maternal Pod and Patch is available across Europe in the Benelux, DACH, UK&I, Nordics, CEE and Iberia markets.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.

Topics
Contacts

Kathy O'Reilly
Philips Global Press Office Tel.: +1 978-221-8919
You are about to visit a Philips global content page
Continue
Silvie Casanova
Philips North America Tel: +1 781 879 0692
You are about to visit a Philips global content page
ContinueMedia assets
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue