Philips is providing an important reminder to customers in the US and Canada about the November 26, 2021 Philips Infa-Therm Transport Mattress recall notification for this product. This recall notification affects units in the US and Canada shipped between April 2019 and November 2021.
As part of this action, Philips contacted affected customers and distributors and instructed them to cease use of and to destroy affected products.
To help ensure ongoing customer awareness of this recall notification, Philips is posting this online notice.
Philips issued notification in November 2021, because changes were made to the product labeling, which were not within the scope of the product’s original 510(k) clearance. These labeling changes were related to maximum temperature, expiration date, and instructions for use. Philips made the business decision to discontinue manufacture and distribution. At the time of the notification, the product was no longer manufactured or distributed by Philips. In January 2022, the FDA designated this notification a Class II recall.
During the 13 years since 2009, Philips has filed four reports of accidental burns in the neonatal population. One of these four reports was later determined not to involve a Philips device, and one of these reports remains under investigation (MDR #1100070000-2022-0001).
If customers need any further information or support concerning this issue, they are advised to email [email protected].
If customers experience any adverse reactions or quality problems with the use of affected products, they may report adverse reactions or quality problems to the FDA’s MedWatch Adverse Event Reporting program either online (https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=consumer.reporting1), by regular mail or by fax (1-800-332-0178).
FAQs
Should customers continue to use their product?
No. Since December 2021, Philips is contacting and instructing customers to destroy affected products in accordance with the recall notification.
How can a customer identify a unit affected by this correction?
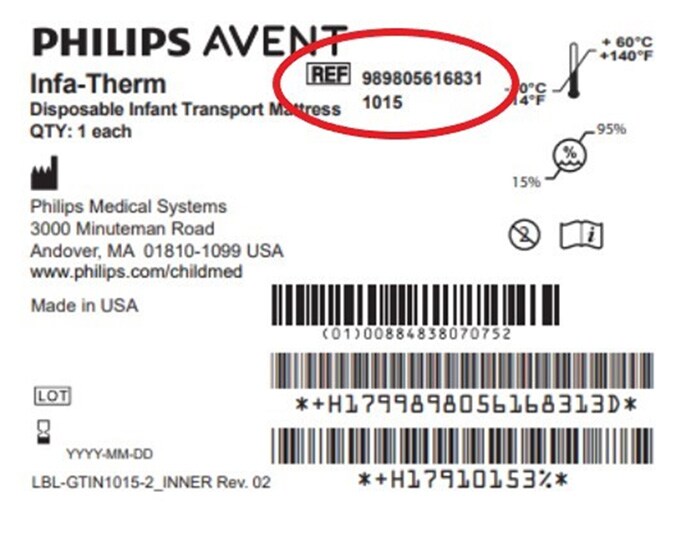
All Philips Infa-Therm Transport Mattress products are impacted. Customers may identify affected products via the following product code reference number: “REF# 989805616831 1015”, located in the top center area of the product label (see red-encircled area in the figure below).
What should customers do if they have devices affected by this correction?
Customers should take the following steps:
Share on social media
Topics
Contact

Mario Fante
Philips Global Press Office Tel: +1 603 560 9226
You are about to visit a Philips global content page
Continue