A comparison of gingivitis and plaque reduction by the Philips Sonicare DiamondClean Prestige 9900 and the Oral-B iO Series 9
Starke M, Nelson M, Foster J, Ward M, Milleman K, Milleman J

Objective
To compare the reduction in gingival inflammation, gingival bleeding and surface plaque by Philips Sonicare 9900 Prestige powered toothbrush and Oral-B iOTM Series 9 powered toothbrush following 6 weeks of home use.
Methodology
Two hundred ninety-five adults (221 females, 74 males) with moderate gingivitis, aged 19-64 years (mean age: 42.3 years), participated in a single- blind, randomized, parallel design, IRB-approved clinical study conducted at a single center. Eligible subjects were regular manual toothbrush users with an average Modified Plaque Index (MPI) score of ³1.8 following three to six hours of plaque accumulation and a Gingival Bleeding Index (GBI) score of ³1 on a minimum 50 sites. Subject scores for MPI, GBI and the Modified Gingival Index (MGI) at baseline were similar. Eligible subjects were randomized to either the Philips Sonicare DiamondClean Prestige 9900 powered toothbrush with the Premium All-in One brush head used in the SenseIQ (Clean) mode (SCP) or the Oral-B iO Series 9 powered toothbrush with the Ultimate Clean brush head in the Daily Clean mode (OB9). Subjects were instructed to brush at home for two-minutes using a timer twice daily for a six-week period in the non-connected mode without the use of the digital smartphone app accessory. Efficacy and safety evaluations occurred at Week 6, in which surface plaque, gingivitis, and bleeding levels were reassessed. Compliance was tracked by subject diary review. Safety was assessed by intraoral exam and subject report.
Results
At Baseline, the MGI Least Squares (LS) mean and 95% Confidence Interval (CI) outcomes were 2.79 (2.75, 2.83) for SCP and 2.77 (2.74, 2.81) for OB9, p-value = 0.5527.
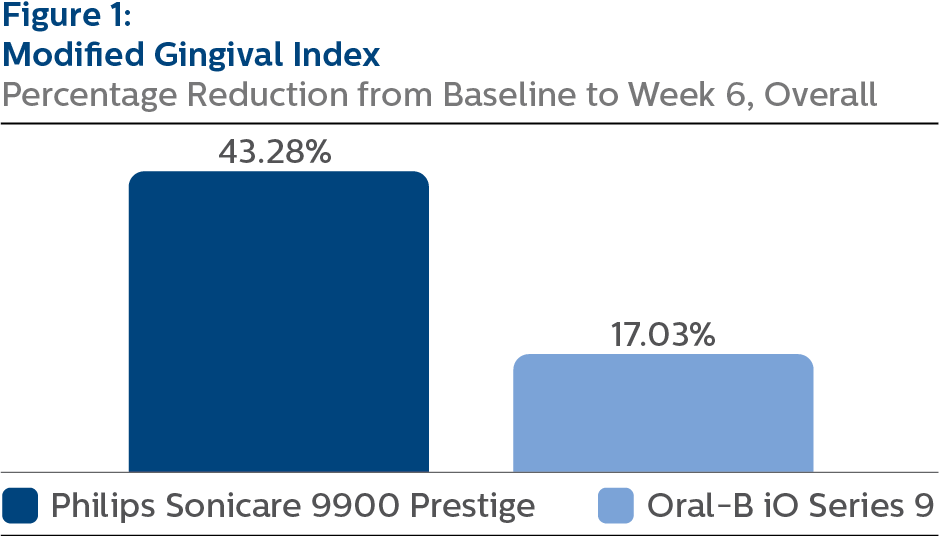
Modified Gingival Index (MGI)
Following six weeks of product use, the LS mean and 95% CI outcomes were 1.58 (1.53, 1.64) for SCP and 2.30 (2.25, 2.36) for OB9, p-value < 0.0001.
Expressed as percent reduction from Baseline overall, this was 43.28% (41.22%, 45.35%) for SCP and 17.03% (14.97%, 19.09%) for OB9, p-value < 0.0001.
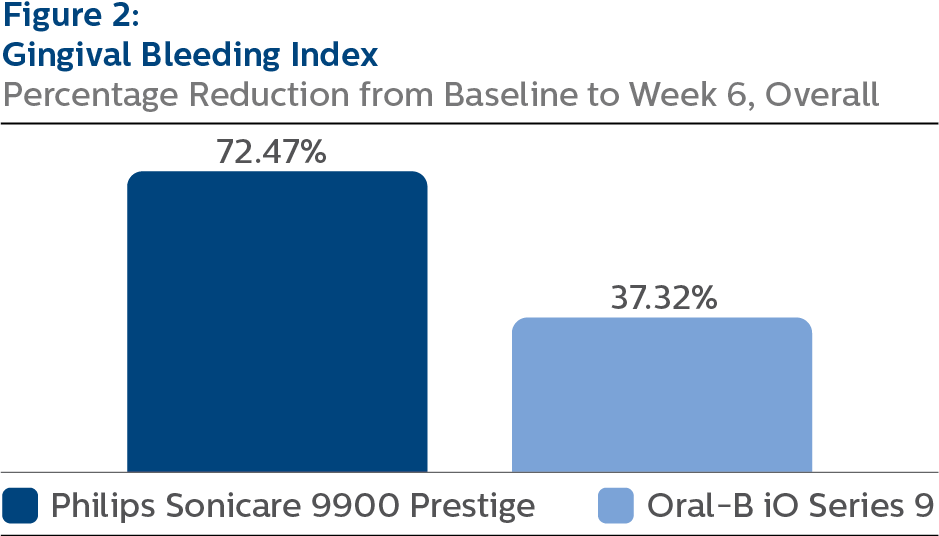
Gingival Bleeding Index (GBI) At Baseline, the GBI LS mean and 95% CI outcomes were 0.51 (0.48, 0.54) for SCP and 0.51 (0.48, 0.53) for OB9, p-value = 0.7208. Following six weeks of product use, the LS mean and 95% CI outcomes were 0.15 (0.13, 0.17) for SCP and 0.32 (0.30, 0.34) for OB9, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 72.47% (68.89%, 76.04%) for SCP and 37.32% (33.76%, 40.88%) for OB9, p-value < 0.0001.
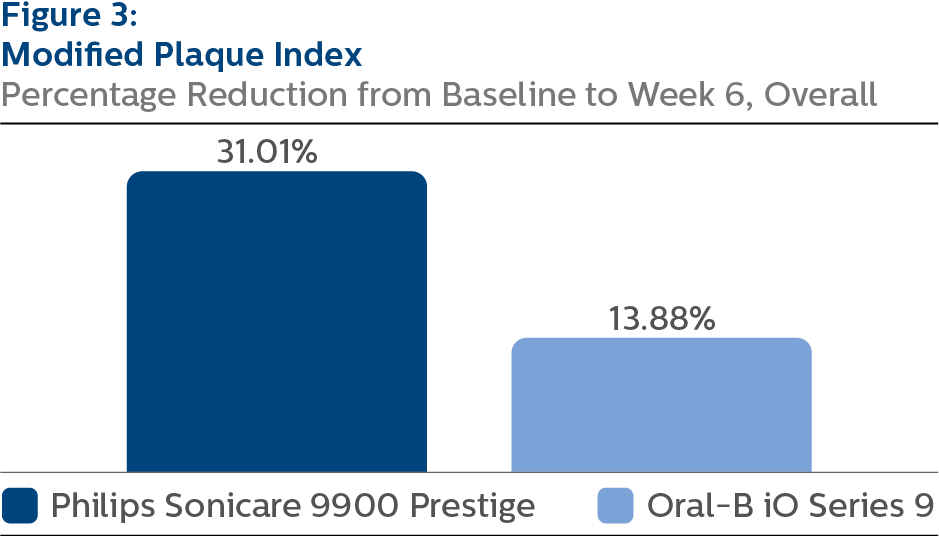
Modified Plaque Index (MPI) At Baseline, the MPI LS mean and 95% CI outcomes were 3.02 (2.97, 3.07) for SCP and 2.97 (2.91, 3.02) for OB9, p-value = 0.1210. Following six weeks of product use, the LS mean and 95% CI outcomes were 2.06 (2.02, 2.11) for SCP and 2.57 (2.52, 2.62) for OB9, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 31.01% (29.42%, 32.61%) for SCP and 13.88% (12.29%, 15.47%) for OB9, p-value < 0.0001.
Gingival Health Using the American Academy of Periodontology / European Federation of Periodontology guidelines where ‘not healthy’ is defined as having ³10% bleeding sites in the whole mouth, all study subjects were ‘not healthy’ at baseline. After six- weeks of home use, 62 (44%) subjects in the SCP group and 3 (2.1%) subjects in the OB9 group, p-value < 0.0001, converted to a ‘healthy’ status with <10% bleeding sites in the whole mouth. The odds of transitioning from ‘not healthy’ at baseline to ‘healthy’ gingivitis status after six weeks was 36 times higher when using SCP than when using OB9. There were four adverse events reported in the study. Three mild ulcers were observed in the SCP group and one self-reported moderate gum irritation in the OB9 group. None of the adverse events were related to the study or study products, except the gum irritation which was deemed possibly related.
Safety
Conclusions
The Philips Sonicare DiamondClean Prestige 9900 powered toothbrush with the Premium All-in-One brush head used in the SenseIQ (Clean) mode was statistically superior to the Oral-B iO Series 9 powered toothbrush with Ultimate Clean brush head used in the Daily Clean mode in reducing gingival inflammation, gingival bleeding and surface plaque following a six-week period of twice-daily home use in a population with moderate gingivitis.
Both products tested are safe for home use.
© 2022 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of Philips Oral Healthcare, LLC. SONICARE and the Sonicare logo are trademarks of Philips Oral Healthcare, LLC.

