Comparison of the reduction in gingivitis and plaque following home-use of Philips Sonicare DiamondClean Smart with Premium Plaque Control brush head and Oral-B Genius 8000 with FlossAction brush head

Objective
To compare the effects of Philips Sonicare DiamondClean Smart with a Premium Plaque Control brush head* and Oral-B Genius 8000® with a FlossAction® brush head on gingival inflammation, gingival bleeding and surface plaque following six weeks of home use.
Methodology
Two hundred nineteen subjects completed this IRB-approved, randomized, single-blind, parallel clinical trial. Informed consent was obtained on all subjects screened for the study.
Eligible subjects were generally healthy manual toothbrush users who were non-flossers and non-smokers. Subjects were to have ≥50 sites of gingival bleeding per Gingival Bleeding Index (GBI) and a plaque score ≥1.8 per Modified Plaque Index (MPI), assessed at 3 to 6 hours following last brushing encounter. Eligible subjects were randomized, with 112 subjects in the Philips Sonicare DiamondClean Smart (DCS) with Premium Plaque Control brush head group, and 107 in the Oral-B Genius 8000® (OBG) with FlossAction® brush head group. Subjects were to use the assigned product for two minutes, twice-daily.
Neither powered toothbrush was used with any active app features. All subjects used a standardized fluoride-containing dentifrice during the home use period and were prohibited from all other oral hygiene measures. Subjects returned to clinic at Week 2 for a compliance and safety check, and at Week 6 for a final assessment of safety and efficacy.
Results
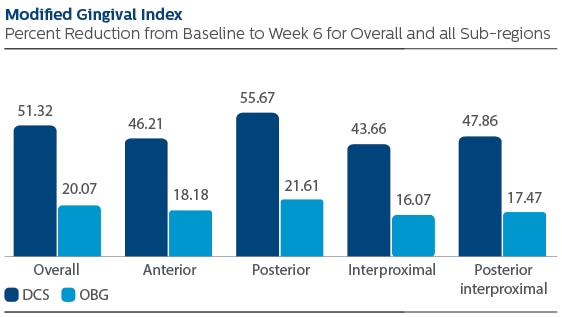
Modified Gingival Index (MGI)
Following six weeks of product use, the Least Squares (LS) mean and 95% Confidence Interval (CI) outcomes were 1.38 (1.30, 1.46) for DCS, and 0.53 (0.45, 0.61) for OBG, p-value < 0.001. Expressed as percent reduction from Baseline overall, this was 51.32% (48.45%, 54.19%) for DCS, and 20.07% (17.14%, 23.00%) for OBG, p-value <0.001.
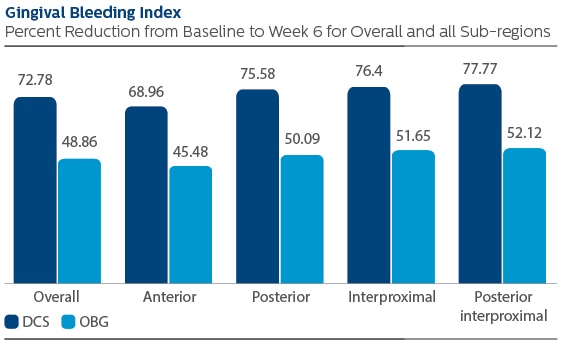
Gingival Bleeding Index (GBI)
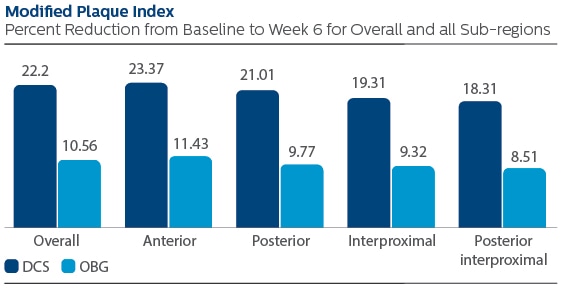
Following six weeks of product use, the LS mean and 95% CI outcomes were 0.42 (0.39, 0.44) for DCS, and 0.29 (0.26, 0.31) for OBG, p-value < 0.001. Expressed as percent reduction from Baseline overall, this was 72.78% (68.95%, 76.60%) for DCS, and 48.86% (44.95%, 52.78%) for OBG, p-value < 0.001. Modified Plaque Index (MPI)
Following six weeks of product use, the LS mean and 95% CI outcomes were 0.67 (0.61, 0.73) for DCS, and 0.32 (0.25, 0.38) for OBG, p-value < 0.001. Expressed as percent reduction from Baseline overall, this was 22.20% (20.08%, 24.31%) for DCS, and 10.56% (8.40%, 12.73%) for OBG, p-value < 0.001.
Safety
There were two adverse events reported. These events were resolved by the end of the study period.
Conclusions
Philips Sonicare DiamondClean Smart with a Premium Plaque Control brush head was statistically significantly superior to Oral-B Genius 8000® with a FlossAction® brush head in reducing gingival inflammation, gingival bleeding and surface plaque following a six-week period of twice-daily home use.
Both products are safe for home use.
*Brush head formerly called AdaptiveClean
© 2019 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV and/or Philips Oral Healthcare, LLC.
Data on file - 10297

