In-vitro plaque removal by Quad Stream and Standard nozzles tested on a Philips Sonicare Cordless Power Flosser
Gottenbos B, Balakrishnan A

Objective
The objective of this study was to determine the levels of in-vitro plaque removal by the Quad Stream and Standard nozzles, when used on a Philips Sonicare Cordless Power Flosser.
Methodology
Dental plaque biofilms were grown from pooled human saliva on enamel disks for four days. The biofilms (n=6 samples per nozzle) were treated with Philips Sonicare Cordless Power Flosser for three seconds at setting 3 in Clean mode. Two nozzles were tested—Quad Stream and Standard. Both nozzles were positioned at a distance of 3 mm from the biofilm sample.
To quantify the number of bacteria before treatment, the biofilm volume was measured using optical coherence tomography (OCT) and the bacterial cell density was determined from untreated control samples (n=6) using confocal laser scanning microscopy (CLSM). After treatment, the number of remaining bacteria was counted using CLSM. From the cell density and the biofilm thickness measurements before and after treatment, the % plaque removal was calculated.
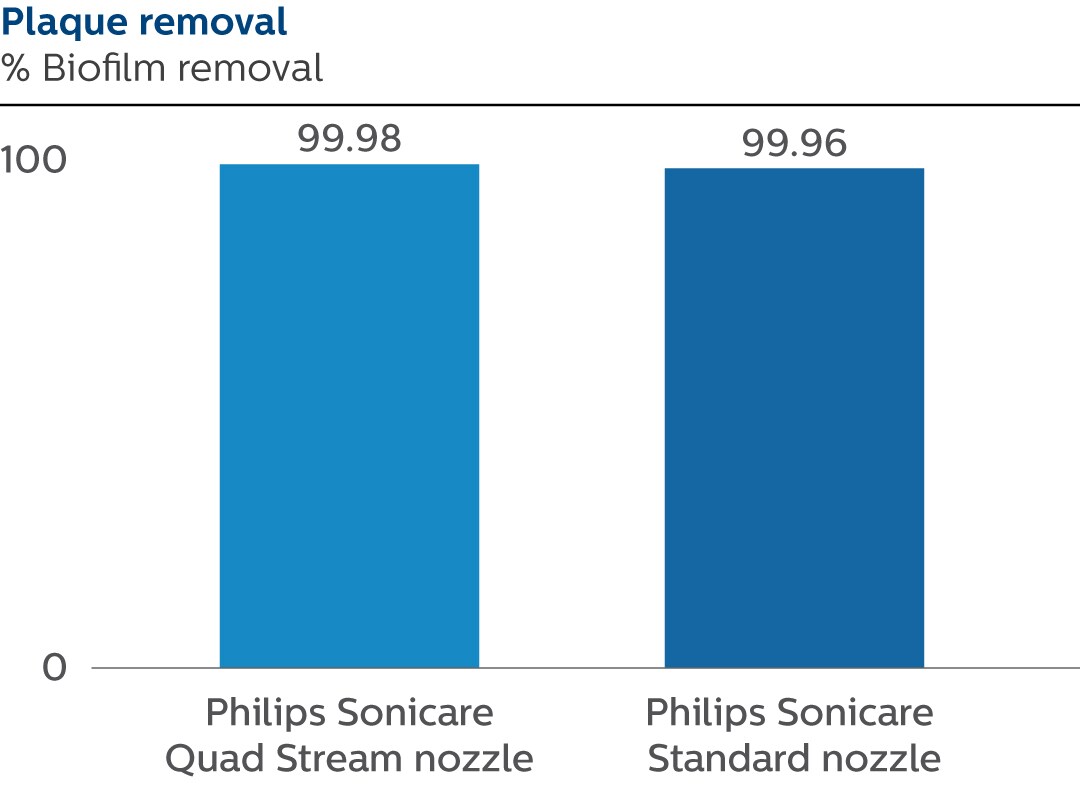
Results
A. With the Quad Stream nozzle, the average biofilm removal measured was 99.98% with a standard deviation of 0.01%. B. With the Standard nozzle, the average biofilm removal measured was 99.96% with a standard deviation of 0.03%.
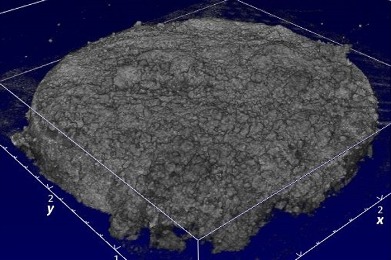
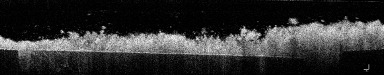
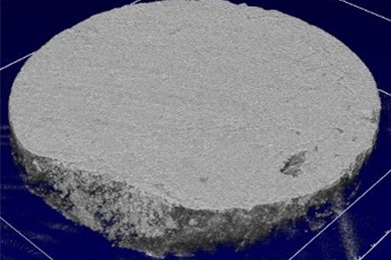
Before treatment, the thickness of plaque biofilm was 0.35 ± 0.16 mm on average. The cell counts in the non-treated biofilm images (77.5 µm x77.5 µm) were 3081 ± 665 (mean ± standard deviation), leading to cell densities of 3.7 ± 0.9 x 108 cells per mm3. Figure 1 shows full 3D OCT scans of a sample before treatment (upper left image, size is 4x4x1 mm) and after treatment with the Quad Stream nozzle (upper right). 2D OCT cross-sections (bottom image, size = 4x1 mm) show that the thickness of the plaque was approximately 0.35 mm before treatment. No plaque biofilm is visible after treatment (bottom right image).
Table 1 shows the plaque removal data. The calculations for biofilm volume removal show that:
Figure 1: 3D OCT scans of enamel samples (untreated on left and treated with Quad Stream nozzle on right)
Before treatment
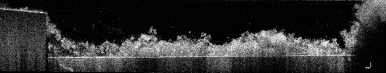
After treatment


| | Biofilm thickness | | Pre # cells/mm2 | | Post # cells/mm2 | | % Biofilm removal | |
| Sample | Quad stream | Standard | Quad stream | Standard | Quad stream | Standard | Quad stream | Standard |
| 1 | 0.334 | 0.201 | 9.17E+07 | 1.24E+08 | 2.34E+04 | 2.47E+04 | 99.974 | 99.980 |
| 2 | 0.297 | 0.175 | 1.84E+08 | 1.10E+08 | 4.78E+04 | 1.01E+05 | 99.974 | 99.908 |
| 3 | 0.405 | 0.308 | 9.18E+07 | 1.50E+08 | 2.97E+04 | 6.55E+03 | 99.968 | 99.996 |
| 4 | 0.248 | 0.310 | 1.15E+08 | 7.43E+07 | 2.63E+04 | 1.89E+04 | 99.977 | 99.975 |
| 5 | 0.496 | 0.777 | 2.88E+08 | 6.47E+07 | 1.53E+04 | 3.57E+04 | 99.995 | 99.945 |
| 6 | 0.248 | 0.450 | 1.67E+08 | 1.14E+08 | 7.46E+03 | 4.50E+04 | 99.996 | 99.961 |
| Average | 0.34 | 0.37 | 1.56E+08 | 1.06E+08 | 2.50E+04 | 3.86E+04 | 99.98 | 99.96 |
| SD | 0.10 | 0.22 | 7.51E+07 | 3.17E+07 | 1.38E+04 | 3.33E+04 | 0.01 | 0.03 |
Conclusions
The in-vitro plaque removal efficacy of Philips Sonicare Cordless Power Flosser, when used with the Quad Stream nozzle and the Standard nozzle, was characterized via OCT/CLSM on a plaque biofilm model. The measured plaque removal by both Quad Stream nozzle and the Standard nozzle was 99.9%.
© 2021 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV.
Data on file - D000864604(1)

