Periodontal pocket cleaning and microbiome changes after using a Cordless Philips Sonicare Power Flosser in vitro
Gottenbos B, Balakrishnan A, van de Kamp-Peeters L, Keijser B

Objective
To assess the effectiveness of the Cordless Philips Sonicare Power Flosser (PSPF) in removing biofilm from an in vitro model periodontal pocket up to 6 mm deep and to investigate the taxonomic composition of biofilm regrown after use of the PSPF relative to a control.
Methodology
Periodontal biofilm was grown on surrogate tooth surfaces, starting from a pooled saliva–plaque mixture using nutrient-supplemented artificial saliva. The tooth surfaces were modeled as a buccal half (n = 10/group) or a distal approximal half (n = 10/group) of adult molars. The surrogate teeth were 3D printed using black polyamide material. Biofilm that resembled the localized conditions within a periodontal pocket was grown on the tooth surfaces under an anaerobic atmosphere followed by a microaerophilic atmosphere. After 48 hours of biofilm growth, the surrogate tooth surfaces were placed in an in vitro model that simulated a row of molars having interproximal spaces and rubber gums shaped to create up to 6 mm deep periodontal pockets. One group of biofilm-coated surfaces was exposed to a cordless PSPF 3000 (clean mode, setting 3) with Quad Stream nozzle according to directions for use. The other group of surfaces was left untreated as a control. The biofilm thickness within the deepest 2 mm of the pocket was analyzed using cross-polarized light microscopy and optical coherence tomography. After assessing biofilm thickness, the surrogate tooth surfaces were once again placed in growth media. After 48 hours of biofilm regrowth, the surrogate surfaces were imaged using confocal laser scanning microscopy with a fluorescent Gram stain. Additionally, after biofilm regrowth the full subgingival part of the biofilm was collected for metagenomic shotgun sequencing. Analysis of the biofilm microbiome after regrowth was analyzed by examining the fractions of the pathogenic red complex bacteria (Porphyromonas gingivalis and Tannerella forsythia) and benign yellow complex bacteria (Streptococcus oralis and Streptococcus gordonii) per the designation of Socransky1.
Results
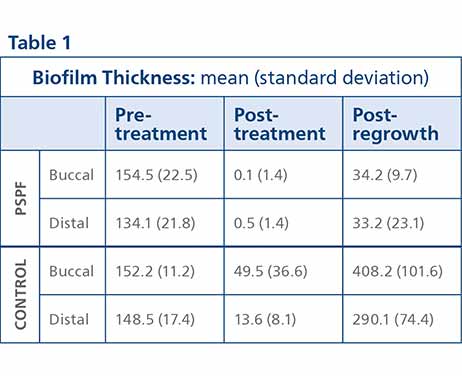
Biofilm Thickness The biofilm thickness was reduced by both treatments (Table 1). The reduction in biofilm thickness was significantly greater for the PSPF group versus the control (p-value <0.002). After 48 hours of biofilm regrowth the biofilm thickness increased. The increase in biofilm thickness was significantly less for the PSPF group versus the control group (p-value <0.001). Figure 1 shows representative images of the buccal samples for the pre-treatment, post-treatment and post-regrowth evaluation points.
Biofilm Microbiome
Analysis of the biofilm microbiome after 48 hours of regrowth showed that Gram-positive cocci colonies were observed within regrown PSPF-treated samples, whereas in the control biofilms mainly Gram-negative rods were present (Figure 1(D)). Metagenomic sequencing detected 281 species after 48 hours of biofilm regrowth, showing the biofilms were diverse. In the PSPF group, the fraction of pathogenic red complex bacteria in the biofilm after regrowth was lower than the fraction in the control group (Table 2). In the PSPF group, the fraction of benign yellow complex bacteria in the biofilm after regrowth was higher than the fraction in the control group.
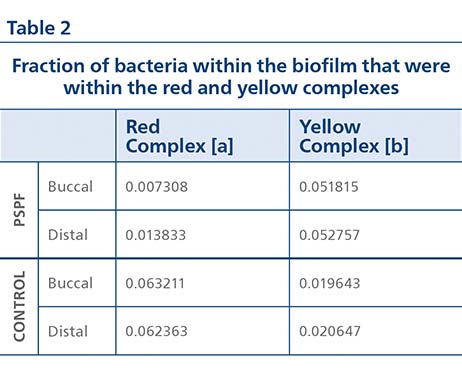
[a] Red complex bacteria represented by P. gingivalis and T. forsythia.
[b] Yellow complex bacteria represented by S. oralis and S. gordonii.
Discussion
As the trends for the buccal and distal surfaces were similar, the following discussion presents the average results for the two tooth surfaces within this in vitro periodontal pocket model.
The results show that treatment with the PSPF resulted in 99% of the biofilm being removed from the pocket as measured by changes in the biofilm thickness before and immediately after treatment. For pocket surfaces treated with the PSPF, the post-regrowth biofilm thickness (after 48 hours) was 75% thinner than the pre-treatment biofilm thickness. Additionally, pockets treated with the PSPF showed 90% less biofilm regrowth (after 48 hours) relative to the control, again as measured by changes in the biofilm thickness.
In the pockets cleaned with the PSPF, benign yellow complex bacteria outnumbered pathogenic red complex bacteria by 5 times, whereas in the pockets not cleaned with PSPF the pathogenic red complex bacteria outnumbered the benign yellow complex bacteria by 3 times. Examining the ratio of yellow complex bacteria to red complex bacteria relative to the control group, pockets cleaned with the PSPF have 17 times as much benign yellow complex bacteria per pathogenic red complex bacteria.
Combining the reduction in biofilm thickness during treatment and the subsequent 48-hour regrowth of biofilm, there was a total 98% reduction in red complex periodontal pathogens within the pocket after use of the PSPF relative to the control group.
Conclusion
Within this in vitro test, the cordless Philips Sonicare Power Flosser 3000 (clean mode, setting 3) with the Quad Stream nozzle was able to reduce the thickness of biofilm by 99% from the deepest sections (4 mm to 6 mm) of a model periodontal pocket. Furthermore, there was a microbiome difference after 48 hours of biofilm regrowth resulting in a lower relative abundance of periodontal pathogens in the power flosser group versus the non-cleaned control group.
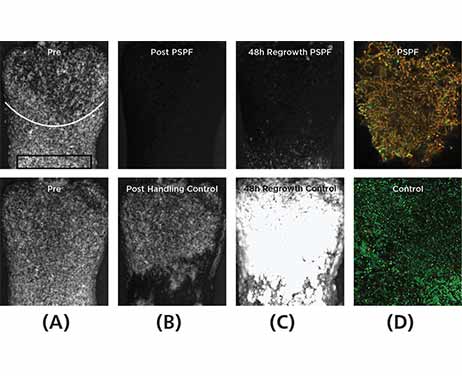
Fig 1. Typical buccal surface images (biofilm = white; tooth = black). The top row of images is from the Philips Sonicare Power Flosser 3000 (clean mode, setting 3) with Quad Stream nozzle (PSPF)–treated group and the bottom row of images is from the control group. (A) Images of the surfaces before placement in the in vitro model with a white arc line marking the gum line and a black rectangle marking the deepest 2 mm of the pocket used for analysis. (B) After PSPF treatment or control (showing effect of handling). (C and D) After 48 hours, biofilm regrowth. Images (A) through (C) are captured with cross-polarized light microscopy and images (D) are captured with confocal laser scanning microscopy (1000x magnification) with an image size of 40 µm x 40 µm. The bacteria of (D) are dyed with a Gram stain: green = Gram-negative bacteria; yellow to red = Gram-positive bacteria.
References:

