An assessment of plaque and gingivitis using a Philips Sonicare 4000-5000 Series* and a manual toothbrush after a four-week period of home use
Starke M, Nelson M, Foster J, Ward M, Milleman K, Milleman J

Objective
To evaluate the ability of the Philips Sonicare toothbrush 4100, 4300, 4900 and 5200 Series with a C2 Optimal Plaque Control brush head to reduce surface plaque, gingivitis and gingival bleeding versus a manual toothbrush, following four weeks of product use.
Methodology
One hundred thirty adults (84 females, 46 males) with mild gingivitis, aged 18-65 years (mean age: 39.6 years), participated in a single-blind, randomized, parallel design, IRB-approved clinical study. Eligible subjects were regular manual toothbrush users with an average Modified Plaque Index (MPI) of ³1.8 following three to six hours of plaque accumulation and a Gingival Bleeding Index (GBI) of ³1 on at least 20 sites. Subject scores for MPI, GBI and the Modified Gingival Index (MGI) at baseline were similar. Eligible subjects were randomized to either Philips Sonicare powered toothbrush* with a C2 Optimal Plaque Control brush head (SC2) or manual toothbrush (MTB) for home use. Subjects were instructed to brush twice daily for a four-week period. Efficacy and safety evaluations occurred at Week 4, in which surface plaque, gingivitis, and bleeding levels were reassessed. Compliance was tracked by subject diary review. Safety was assessed by intraoral exam and subject report.
Results
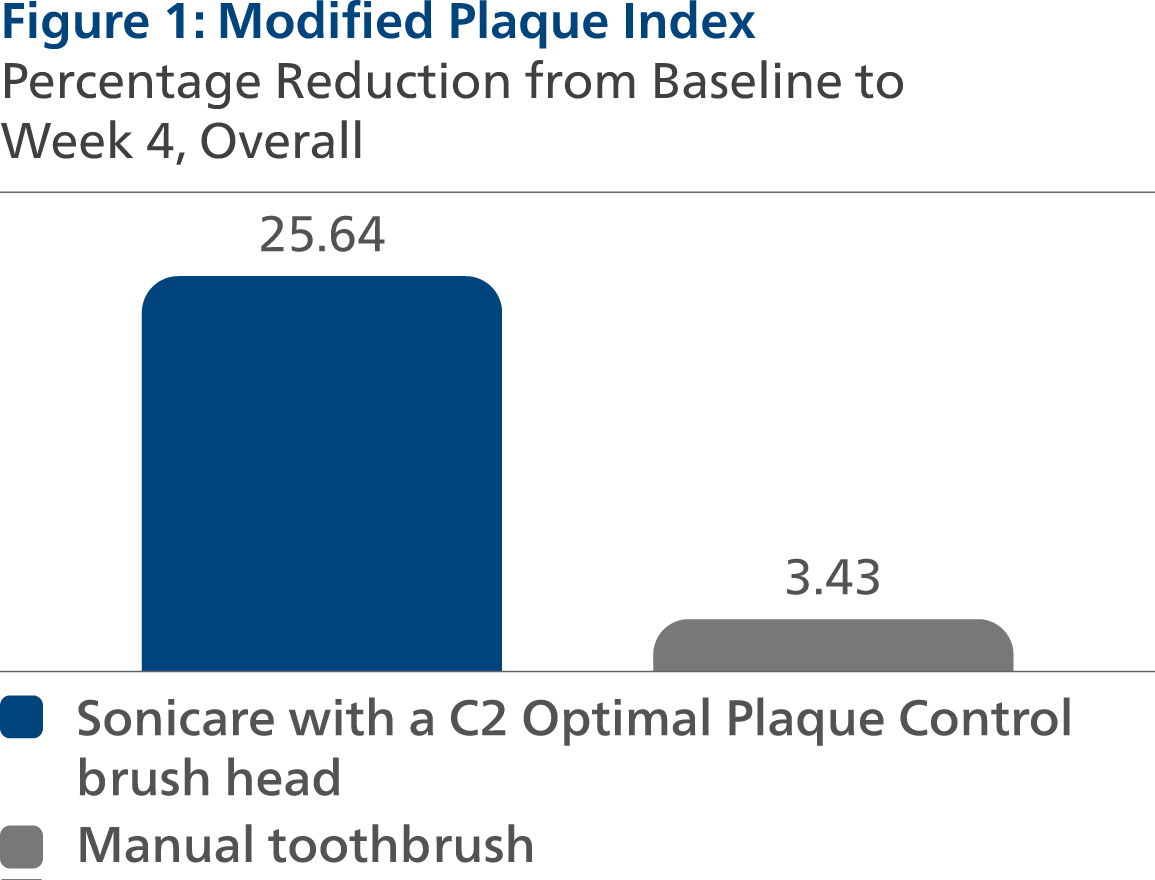
Following four weeks of product use, the Least Squares (LS) mean and 95% Confidence Interval (CI) outcomes were 2.15 (2.09, 2.21) for SC2, and 2.79 (2.73, 2.85) for MTB, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 25.64% (23.48%, 27.80%) for SC2 and 3.43% (1.30%, 5.55%) for MTB, p-value < 0.0001.
Modified Plaque Index (MPI)
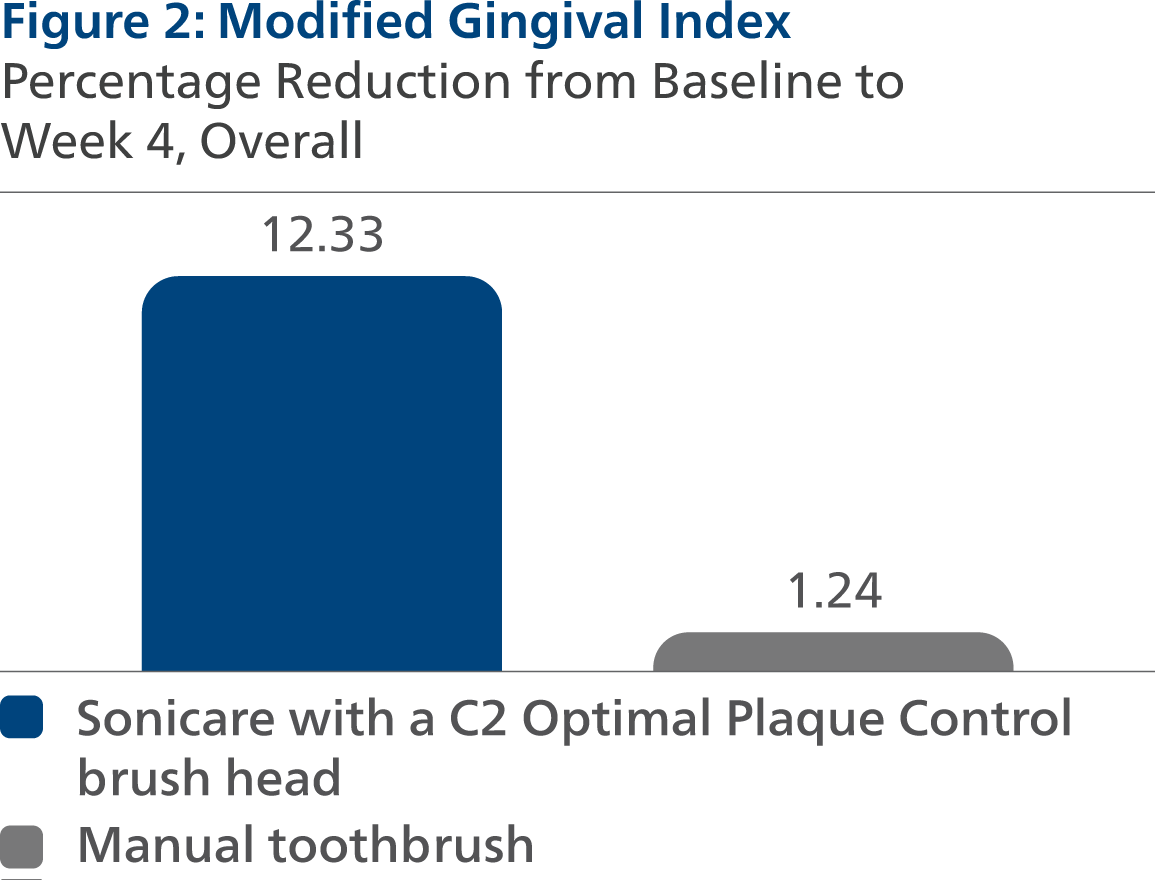
Modified Gingival Index (MGI) Following four weeks of product use, the LS mean and 95% CI outcomes were 2.19 (2.15, 2.23) for SC2, and 2.47 (2.43, 2.51) for MTB, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 12.33% (10.77%, 13.90%) for SC2 and 1.24% (-0.30%, 2.78%) for MTB, p-value < 0.0001.
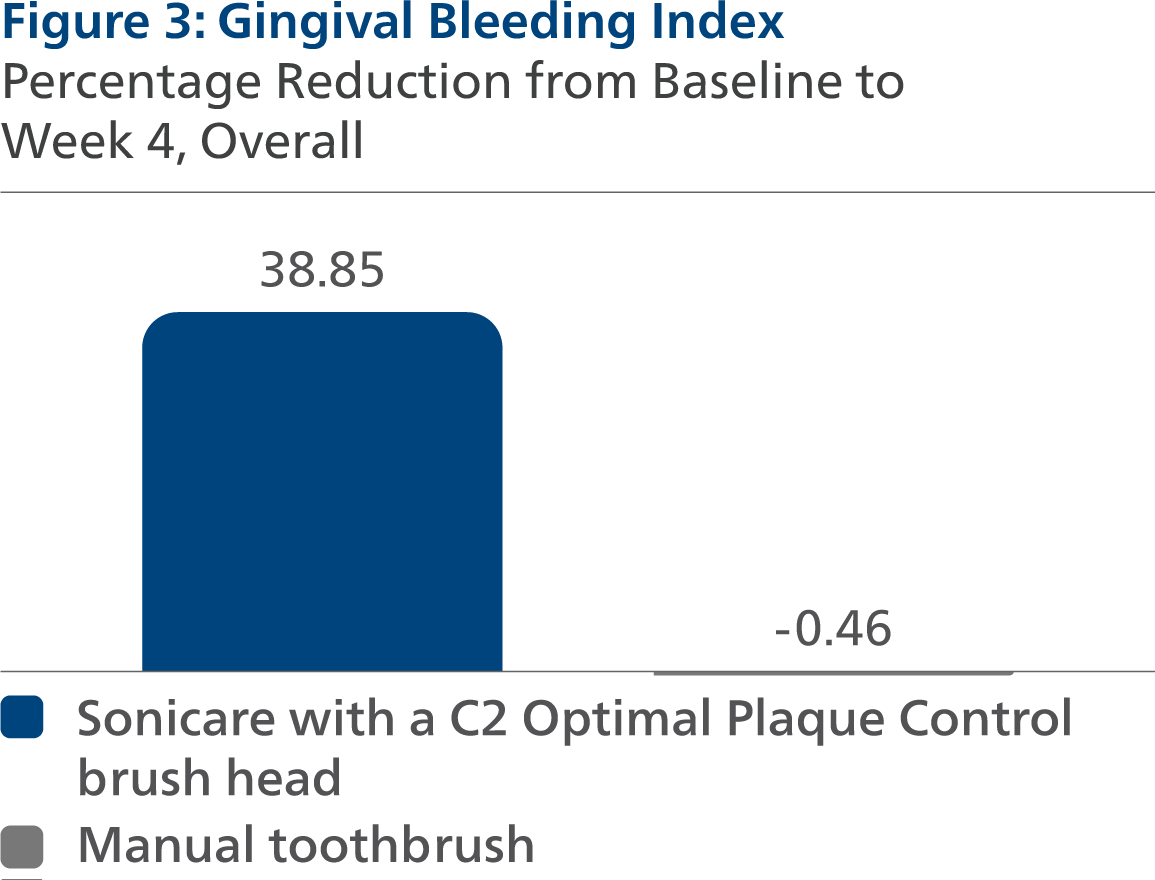
Gingival Bleeding Index (GBI) Following four weeks of product use, the LS mean and 95% CI outcomes were 0.20 (0.18, 0.22) for SC2, and 0.33 (0.31, 0.35) for MTB, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 38.85% (33.03%, 44.66%) for SC2 and -0.46% (-6.18%, 5.27%) for MTB, p-value < 0.0001.
Safety There were four adverse events reported during the study: three unrelated to product use and one mild gingival abrasion within the MTB group.
Conclusions
The Philips Sonicare 4000-5000 Series* powered toothbrush with a C2 Optimal Plaque Control brush head was statistically superior to a manual toothbrush in reducing surface plaque, gingival inflammation and gingival bleeding following a four-week period of twice-daily home use.
Products referenced within are safe for home use.
* Philips Sonicare 4100 Series, 4300 Series, 4900 Series and 5200 Series
© 2022 Koninklijke Philips N.V. (KPNV). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV.
Data on file - DRC OHC_11313

