A comparison of the effects of the Philips Sonicare DiamondClean Prestige 9900 power toothbrush with A3 Premium All-in-One brush head versus a manual toothbrush on plaque levels and gingival health
Milleman J, Milleman K, Ward M, Starke M, Nelson M, Ambers J

Objective
The objective of this study was to evaluate the effects of twice-daily powered toothbrushing, compared to manual toothbrushing, on surface plaque, gingival bleeding and gingival inflammation, following a six-week period of home use, in a population exhibiting moderate gingivitis
Materials and methods
This was an IRB-approved, single-blind, randomized, parallel clinical trial conducted in a generally healthy population of adults in the United States. Eligible subjects were aged 18–75 years, non-smokers and habitual manual toothbrush users. Subjects were to have a minimum plaque score of at least 1.8 as scored by the Modified Plaque Index (MPI), following a three-to six-hour plaque accumulation period, as well as a gingival bleeding score of 1.0 on at least 50 sites, as scored by the Gingival Bleeding Index (GBI). Approximately 110 subjects (55 per treatment group) were to be randomized in order to complete with 98 evaluable subjects (49 per group).
Eligible subjects were randomized to one of two treatment groups: twice-daily powered toothbrushing using Philips Sonicare DiamondClean Prestige 9900 with A3 Premium All-in-One brush head (PSP) or twice-daily manual toothbrushing (MTB) using a Colgate Classic adult toothbrush. All subjects were dispensed the same fluoride-containing dentifrice. The use of any other oral hygiene product or procedure was not permitted during the study period. Following eligibility and randomization at baseline, subjects were seen in clinic at Week 2 and Week 6 for evaluation of efficacy parameters (MPI, GBI and Modified Gingival Index (MGI)), as well as for safety assessment. Subjects were provided a diary card to document compliance and to report any safety events as a result of participation in the study.
Results
There were 104 subjects screened and randomized, 52 subjects in the PSP group and 52 in the MTB group. Of these, 99 subjects completed the study. The mean age of all randomized subjects was 40.7 (SD=10.8) years. There were 78 (75%) females and 26 (25%) males and the majority of the subjects were white: 98 (94.2%).
At baseline, plaque (p-value = 0.40), gingival inflammation (p-value = 0.58), and gingival bleeding (p-value = 0.65) levels were similar for both PSP and MTB groups.
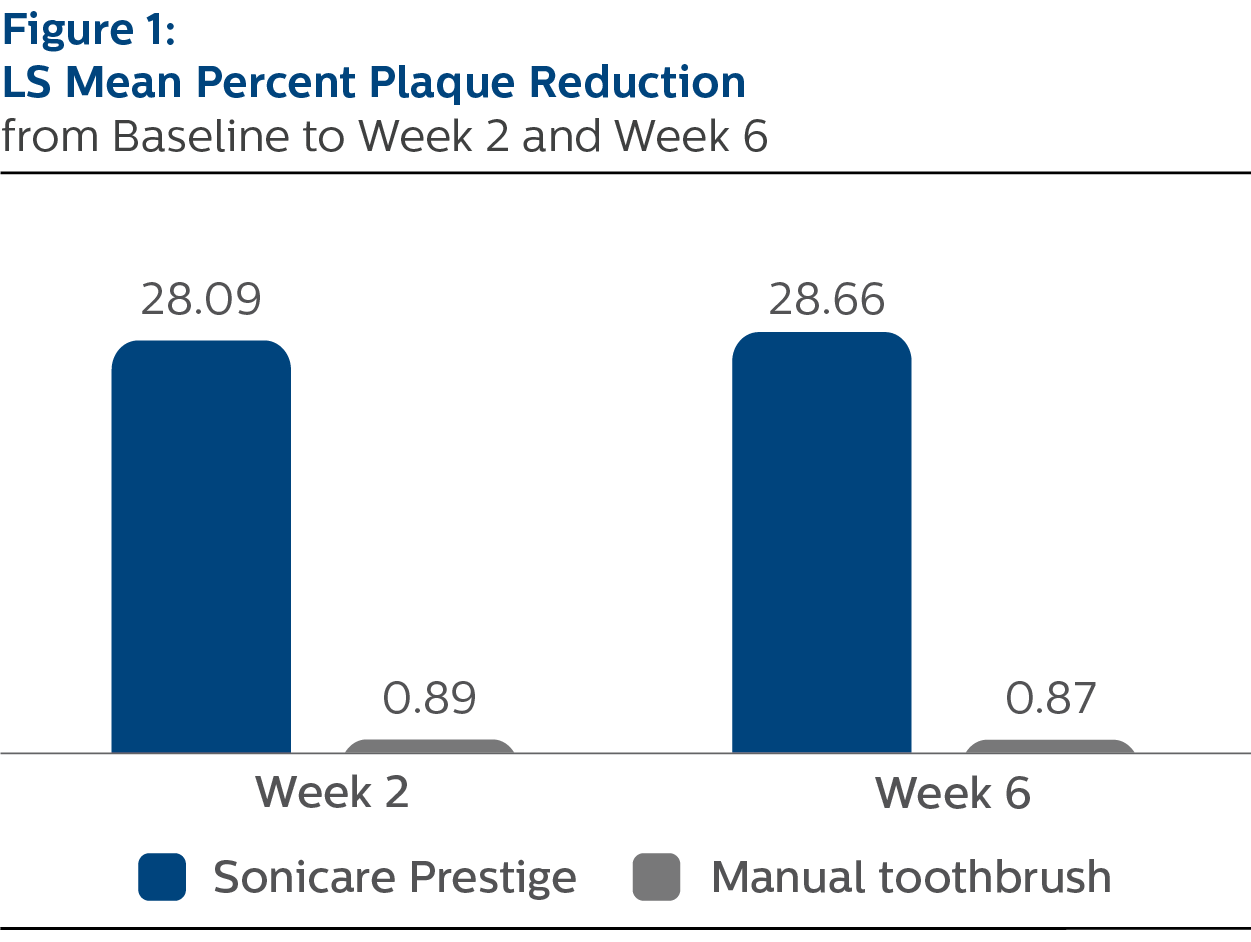
Modified Plaque Index
For percent reduction from baseline in plaque levels following two weeks of assigned product use, the LS mean (SE) outcomes were 28.09% (1.38) for PSP and 0.89% (1.36) for MTB, p-value <.0001.
At Week 6, the percent reduction values were 28.66% (1.37) for PSP and 0.87% (1.36) for MTB, p-value <.0001.
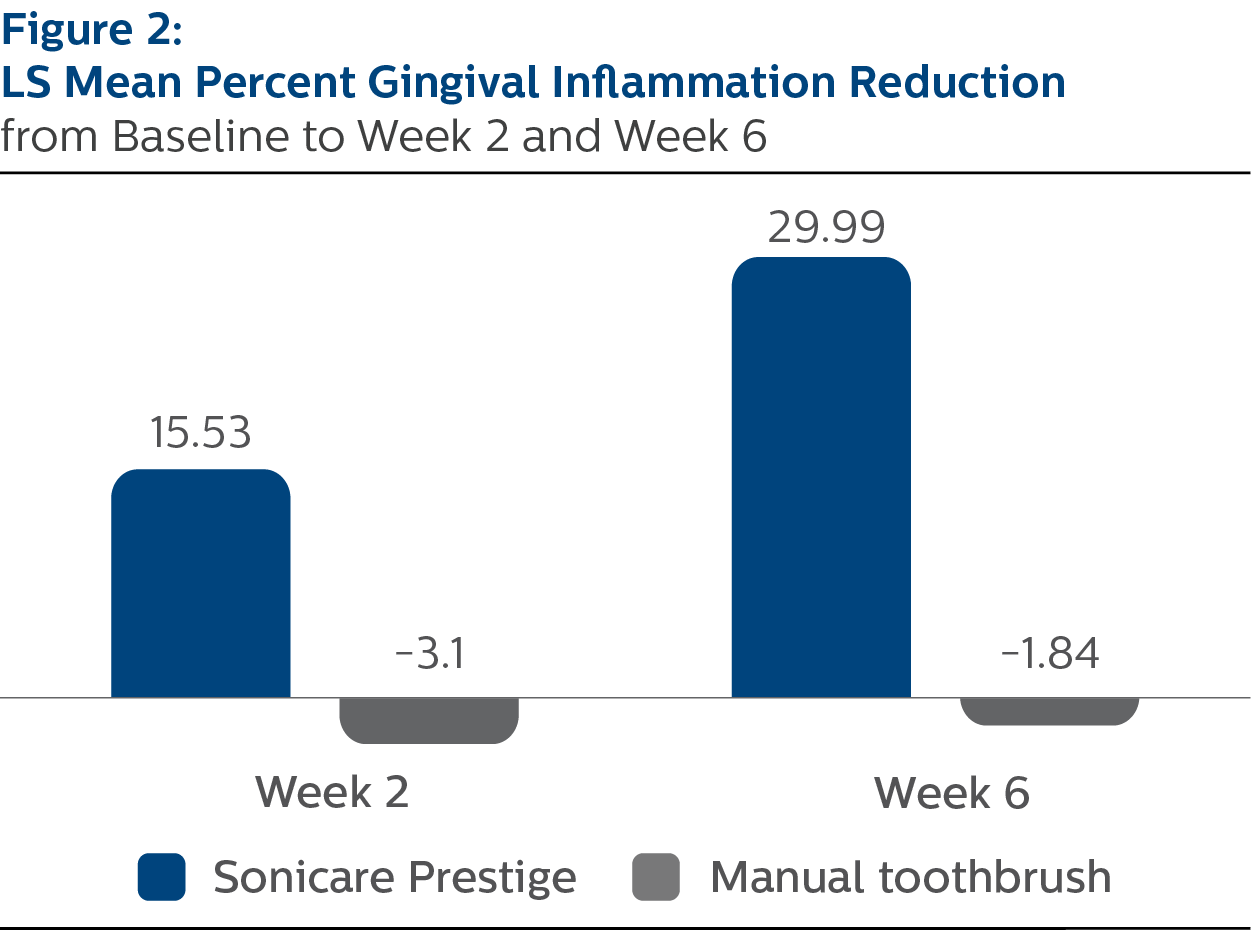
Modified Gingival Index
For percent reduction from baseline in gingival inflammation levels following two weeks of assigned product use, the LS mean (SE) outcomes were 15.53% (1.27) for PSP and -3.10% (1.26) for MTB, p-value <.0001.
At Week 6, the percent reduction values were 29.99% (1.68) for PSP and -1.84% (1.67) for MTB, p-value <.0001.
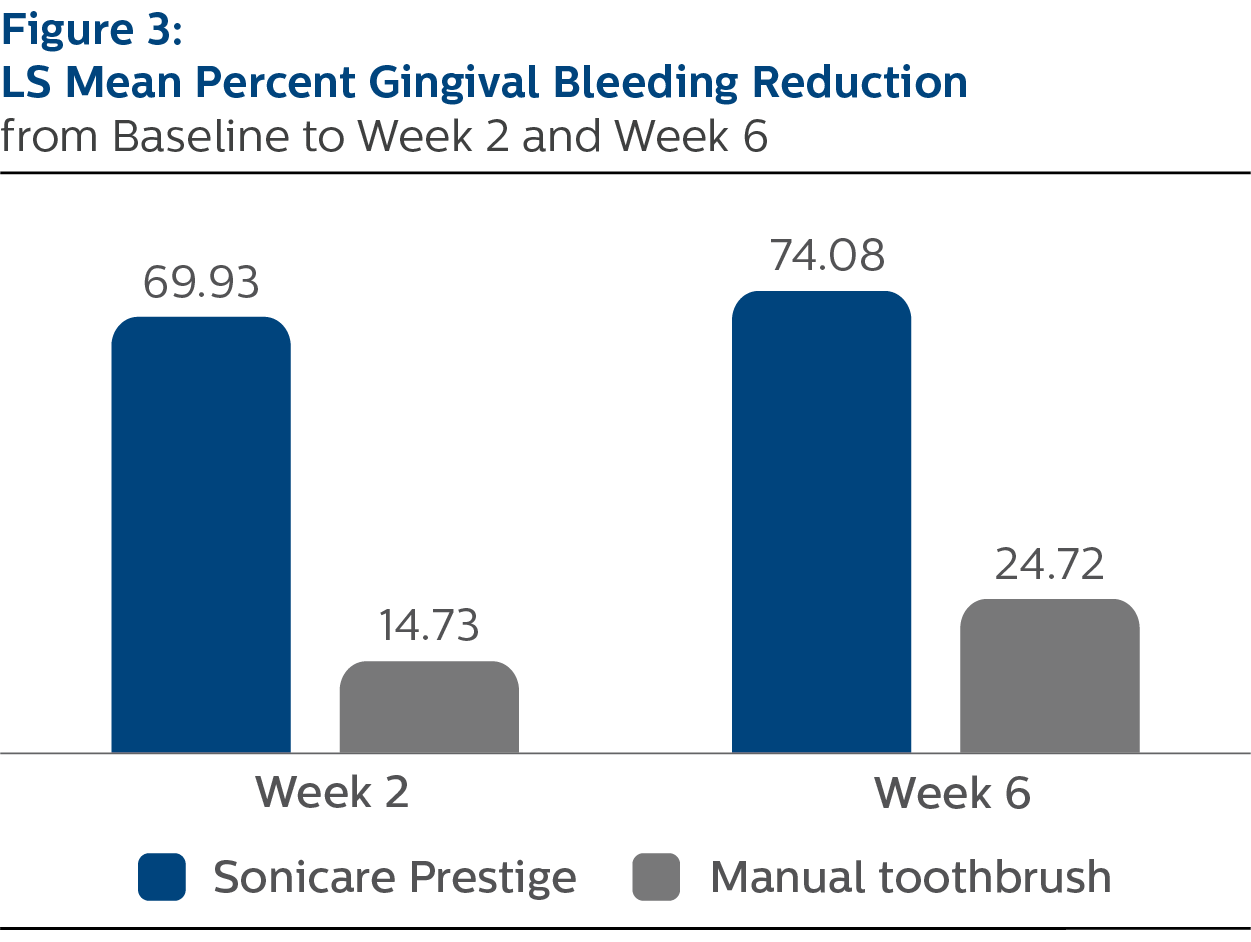
Gingival Bleeding Index
For percent reduction from baseline in gingival bleeding following two weeks of assigned product use, the LS mean (SE) outcomes were 69.93% (3.29) for PSP and 14.73% (3.26) for MTB, p-value <.0001.
The proportion of responders* at Week 2 for PSP was 27 out of 50 subjects, or 54% of subjects.
There were no responders in the MTB group. This difference was statistically significant, p-value < 0.0001.
At Week 6, the percent reduction values were 74.08% (2.64) for PSP and 24.72% (2.61) for MTB, p-value <.0001.
The proportion of responders* at Week 6 for PSP was 23 out of 49 subjects, or 46.9% of subjects. There were no responders in the MTB group. This difference was statistically significant, p-value < 0.0001.
Safety and compliance
There were four reported adverse events, three in the MTB group and one in the PSP group. None of the reported events were related to study product use.
There were nine subjects with at least one protocol deviation and no subjects with significant deviations warranting study withdrawal. The reported deviations included missed brushing or brushing outside the prescribed timing window prior to clinic visits.
Conclusions
In a population of subjects with moderate gingivitis, use of the Philips Sonicare DiamondClean 9900 power toothbrush and A3 Premium All-in-One brush head resulted in statistically significant reductions in surface plaque, gingival bleeding and gingival inflammation, following two and six weeks of use, compared to manual toothbrushing.
Both products were safe for use.
*As per the 2017 published criteria following the World Workshop conducted by the American Academy of Periodontology and the European Federation of Periodontology (https://doi.org/10.1002/JPER.17-0719), a patient’s periodontal status is deemed ‘not healthy’ when > 10% of sites exhibit gingival bleeding. In this study population, those subjects who are characterized as ‘responders’ are those who transitioned from not healthy (i.e., >10% bleeding sites at baseline) to healthy (<10% bleeding sites post baseline).
© 2021 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV.
Data on file - OHC_10863

