Comparison of Plaque and Gingivitis Reduction by Philips Sonicare FlexCare Platinum with Premium Plaque Control Brush Head and a Manual Toothbrush
Jenkins W, Souza S, Ward M, Defenbaugh J, Milleman KR, Milleman JL. J Clin Dent 2017;28(Spec Iss A):A7-12.

Objective
To compare the effects of Philips Sonicare FlexCare Platinum with Premium plaque control* brush head and an ADA reference manual toothbrush on plaque and gingivitis following two and six weeks of home use.
Methodology
One hundred fifty-four adults (mean age 40.62; 111 female/43 male) were consented, enrolled and randomized in this IRB-approved, single-center, examiner-blinded, parallel-design clinical trial.
One hundred forty-three subjects completed the study. Eligible subjects were routine manual toothbrush users who were non-smokers, aged 18-65 with a minimum plaque score of ≥1.8 per Lobene and Soparker Modified Plaque Index (MPI) following 3-6 hours of plaque accumulation, and a Gingival Bleeding Index (GBI) of ≥1 on at least 20 sites.
Eligible subjects were randomly allocated to utilize either a Philips Sonicare FlexCare Platinum with Premium plaque control brush head in Deep Clean mode and high intensity twice daily, or an ADA reference manual toothbrush twice daily per their usual technique. Gingivitis (GBI and Modified Gingival Index (MGI)) and MPI efficacy metrics were assessed at Baseline, and following two and six weeks of home use of the study products. Subjects presented to clinic for all visits with 3-6 hours of plaque accumulation. Safety was assessed by intraoral exam and per subject report.
Results
There were two adverse events reported, both of which were reported as unlikely related to the study.
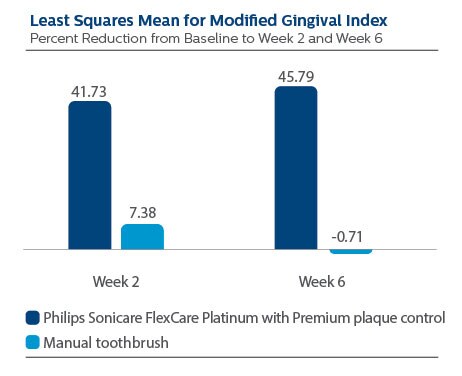
Gingival Inflammation per Modified Gingival Index (MGI)
At Baseline, the MGI LS Mean (SE) outcome for Philips Sonicare FlexCare Platinum with Premium plaque control brush head was 2.16 (0.05), and for manual toothbrush it was 2.27 (0.05), p-value = 0.1282.
Following two weeks of product use, LS Mean (SE) for the Sonicare group was 1.32 (0.04), and for manual toothbrush it was 2.05 (0.04), p-value <0.0001. Expressed as percent reduction versus Baseline, this is 41.73% reduction for Philips Sonicare FlexCare Platinum with Premium plaque control brush head, and 7.38% for manual toothbrush.
Following six weeks of product use, LS Mean (SE) for the Sonicare group was 1.23 (0.04), and for manual toothbrush it was 2.22 (0.04), p-value <0.0001. Expressed as percent reduction versus Baseline, this is 45.79% reduction for Philips Sonicare FlexCare Platinum with Premium plaque control brush head, and -0.71% for manual toothbrush.
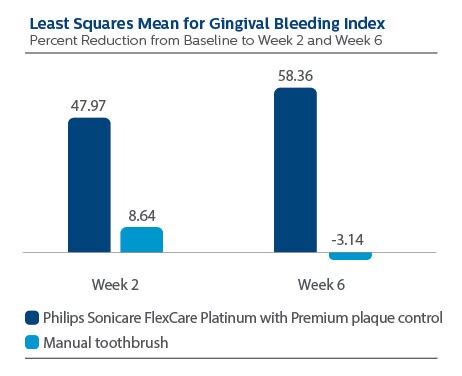
Gingival Bleeding per Gingival Bleeding Index (GBI)
At Baseline, the GBI LS Mean (SE) outcome for Philips Sonicare FlexCare Platinum with Premium plaque control brush head was 0.40 (0.03), and for manual toothbrush it was 0.39 (0.03), p-value = 0.7934.
Following two weeks of product use, LS Mean (SE) for the Sonicare group was 0.19 (0.01), and for manual toothbrush it was 0.34 (0.01), p-value <0.0001. Expressed as percent reduction versus Baseline, this is 47.97% reduction for Philips Sonicare FlexCare Platinum with Premium plaque control brush head, and 8.64% for manual toothbrush.
Following six weeks of product use, LS Mean (SE) for the Sonicare group was 0.15 (0.01), and for manual toothbrush it was 0.38 (0.01), p-value <0.0001. Expressed as percent reduction versus Baseline, this is 58.36% reduction for Philips Sonicare FlexCare Platinum with Premium plaque control brush head, and -3.14% for manual toothbrush.
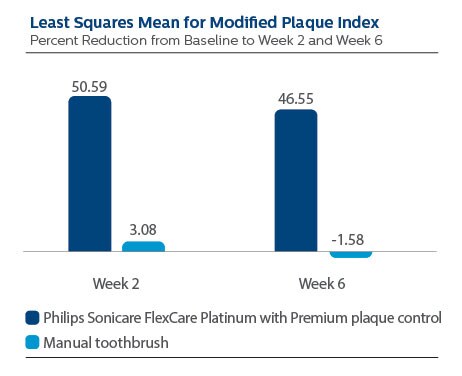
Surface Plaque per Modified Plaque Index (MPI)
At Baseline, the MPI LS Mean (SE) outcome for Philips Sonicare FlexCare Platinum with Premium plaque control brush head was 2.84 (0.06), and for manual toothbrush it was 2.90 (0.06), p-value = 0.4159.
Following two weeks of product use, LS Mean (SE) the Sonicare group was 1.42 (0.06), and for manual toothbrush it was 2.77 (0.06), p-value <0.0001. Expressed as percent reduction versus Baseline, this is 50.59% reduction for Philips Sonicare FlexCare Platinum with Premium plaque control brush head, and 3.08% for manual toothbrush.
Following six weeks of product use, LS Mean (SE) for the Sonicare group was 1.55 (0.07), and for manual toothbrush it was 2.91 (0.07), p-value <0.0001. Expressed as percent reduction versus Baseline, this is 46.55% reduction for Philips Sonicare FlexCare Platinum with Premium plaque control brush head, and -1.58% for manual toothbrush.
Safety
Conclusions
Philips Sonicare FlexCare Platinum with Premium plaque control brush head was statistically superior to an ADA reference manual toothbrush in reducing gingivitis, gingival bleeding and surface plaque following two and six weeks of home use.
Both products were safe for home use.
*Brush head formerly called AdaptiveClean © 2017 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV and/or Philips Oral Healthcare, LLC.
Data on file - MAH-15-0181

