A study to assess the effects of adjunctive use of Philips Sonicare Power Flosser on plaque and gingival inflammation
Salus Research, Inc. Ft. Wayne, IN, USA. Data on file, 2019

Objective
To evaluate the effects of Philips Sonicare Power Flosser, as an adjunct to manual toothbrushing, on plaque and gingivitis following two and six weeks of home use, in comparison to three other oral hygiene regimens.
Methodology
This was a randomized, parallel-design, single-center study. Eligible subjects were aged 18-65 years, non-smokers and routine manual toothbrush users. Subjects exhibited at least moderate levels of gingivitis upon study entry, with a Gingival Bleeding Index (GBI) ³ 1 on a minimum of 50 sites. Additionally, subjects had a minimum plaque score per Rustogi Modified Navy Plaque Index (RMNPI) ³ 0.6 following 3-6 hours plaque accumulation.
Four treatment groups were as follows: ADA reference manual toothbrush (MTB) alone, MTB plus string floss (Reach unflavored waxed floss), MTB plus Philips Sonicare Power Flosser with Quad Stream nozzle (PFQS), and Philips Sonicare ExpertClean power toothbrush with Premium Plaque Control brush head (PTB) plus PFQS. Enrolled subjects were dispensed study products and associated instructions per randomization. They were to perform toothbrushing twice daily, and oral irrigation or flossing (per assignment) once daily (evening). All subjects were dispensed standard fluoride-containing dentifrice. The use of any other oral hygiene products was prohibited during the study period. Efficacy metrics included Modified Gingival Index (MGI), GBI, RMNPI, and were evaluated by a blinded study examiner at Baseline, and at Week 2 and Week 6 following initiation of product use. Safety was assessed by intraoral exam and per subject report.
Results
In total, 260 subjects were enrolled and randomized with 256 subjects who completed the study. The Mean (SD) age of subjects was 41.3 (12.6) years. The study population was 73.8% women.
Demographics
Efficacy
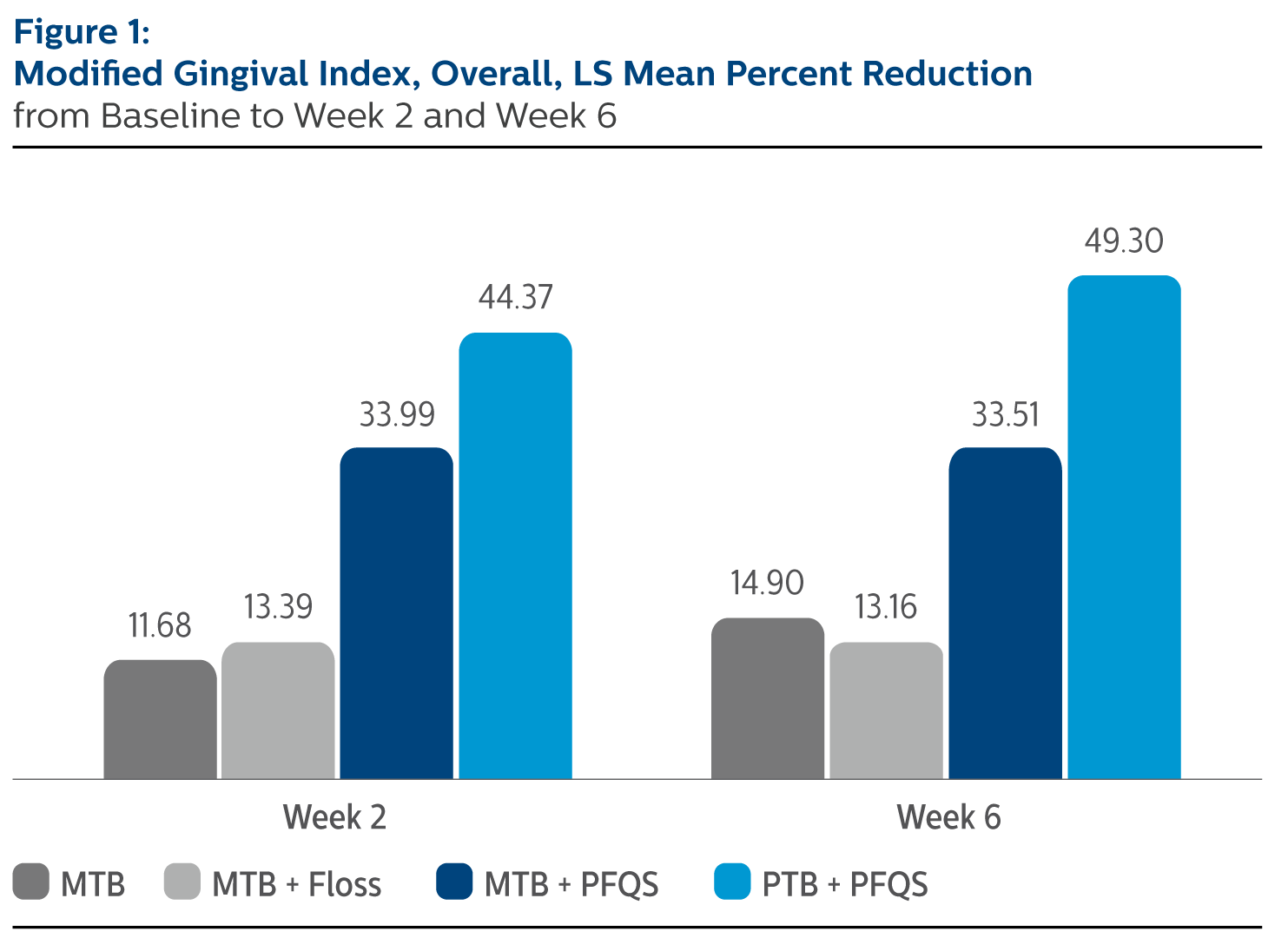
Modified Gingival Index (MGI), Least Square Means (SE), Overall
| | MTB Alone | MTB + Floss | MTB + Power Flosser | Sonicare PTB + Power Flosser |
| Baseline | 2.68 (0.03) | 2.65 (0.03) | 2.66 (0.03) | 2.66 (0.03) |
| Percent Reduction from Baseline | | | | |
| Week 2 | 11.68% (1.95) | 13.39% (1.93) | 33.99% (1.95)*^ | 44.37% (1.95)*^ |
| Week 6 | 14.90% (2.44) | 13.15% (2.40) | 33.51% (2.42)*^ | 49.30% (2.42)*^ |
^Pairwise comparisons were statistically significant versus MTB + Floss treatment group
*Pairwise comparisons were statistically significant versus MTB treatment group
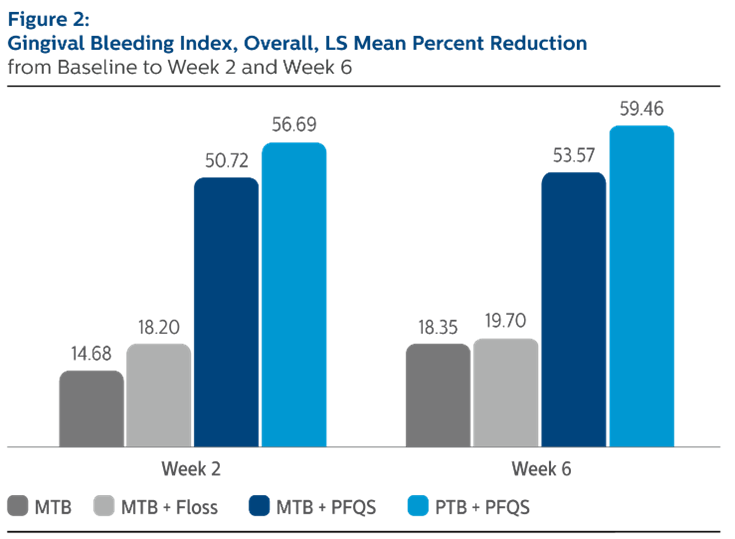
Modified Bleeding Index (MGI), Least Square Means (SE), Overall
| | MTB Alone | MTB + Floss | MTB + Power Flosser | Sonicare PTB + Power Flosser |
| Baseline | 0.54 (0.03) | 0.51 (0.03) | 0.51 (0.03) | 0.54 (0.03) |
| Percent Reduction from Baseline | | | | |
| Week 2 | 14.68% (3.04) | 18.20% (3.02) | 50.72% (3.04)*^ | 59.46% (3.04)*^ |
| Week 6 | 18.35% (3.49) | 19.70% (3.43) | 55.71% (3.46)*^ | 59.46% (3.46)*^ |
^Pairwise comparisons were statistically significant versus MTB + Floss treatment group
*Pairwise comparisons were statistically significant versus MTB treatment group
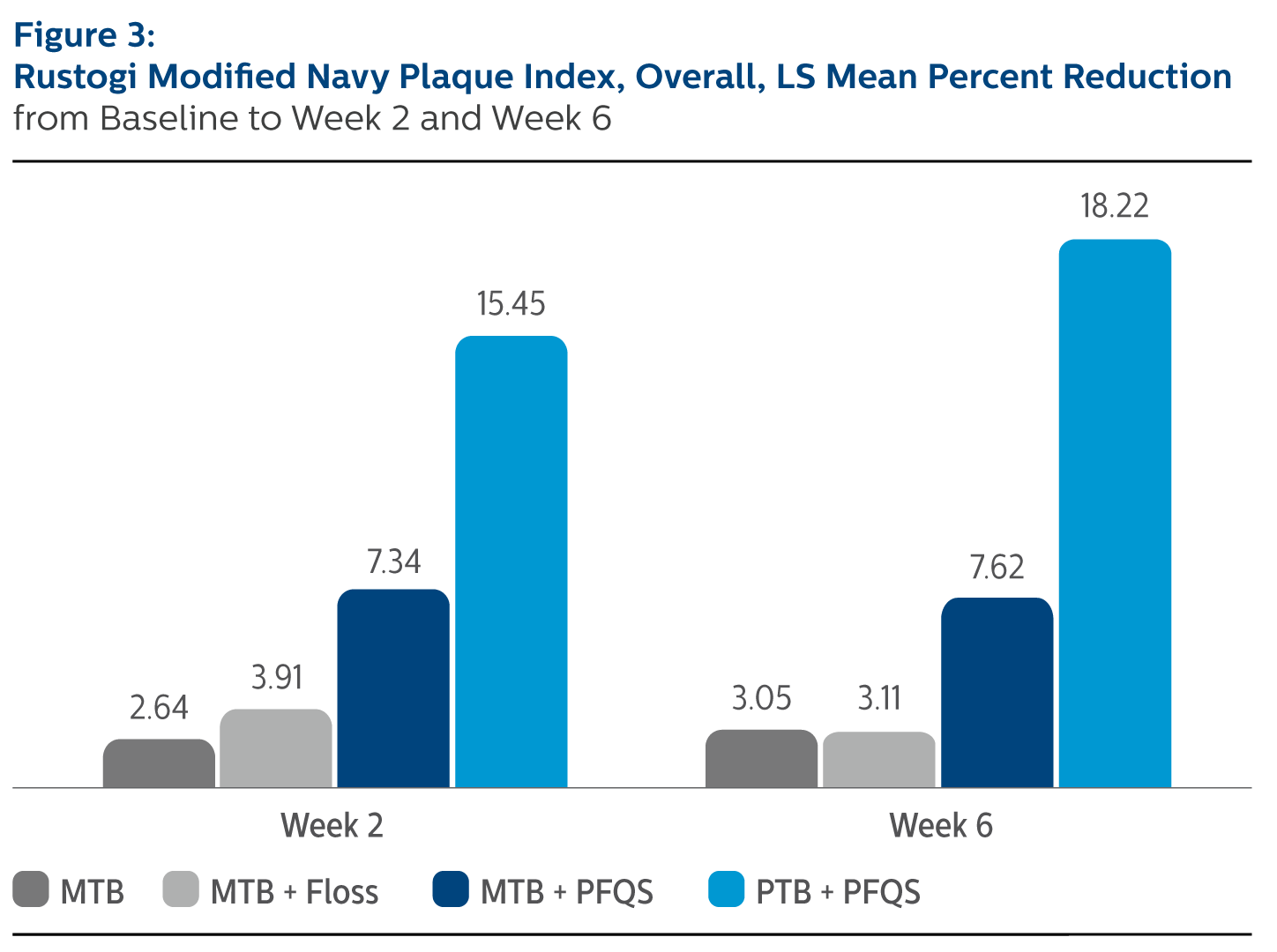
Rustogi Modified Navy Plague Index (RMNPI), Least Square Means (SE), Overall
| | MTB Alone | MTB + Floss | MTB + Power Flosser | Sonicare PTB + Power Flosser |
| Baseline | 0.83 (0.01) | 0.84 (0.01) | 0.83 (0.01) | 0.83 (0.01) |
| Percent Reduction from Baseline | | | | |
| Week 2 | 2.64% (1.03) | 3.91% (1.02) | 7.34% (1.03)*^ | 15.45% (1.03)*^ |
| Week 6 | 3.05% (1.10) | 3.11% (1.09) | 7.62% (1.09)*^ | 18.22% (1.09)*^ |
^Pairwise comparisons were statistically significant versus MTB + Floss treatment group Safety There were three adverse events reported, one each in the MTB alone, MTB plus Floss and MTB plus Power Flosser treatment groups. All the three events were mild, and were not serious.
*Pairwise comparisons were statistically significant versus MTB treatment group
Conclusions
Adjunctive use of the Philips Sonicare Power Flosser with either a manual or powered toothbrush was shown to provide statistically significant benefits in the reduction of gingival inflammation and gingival bleeding, compared to manual toothbrushing alone or manual toothbrush plus floss, following two weeks of home use.
In addition, adjunctive use of the Philips Sonicare Power Flosser with either a manual or powered toothbrush was shown to provide statistically significant benefits in the reduction of gingival inflammation, gingival bleeding and plaque, compared to manual toothbrushing alone or manual toothbrush plus floss, following six weeks of home use.
All products used in the study were safe for home use.
* Philips Sonicare 4100 Series, 4300 Series, 4900 Series and 5200 Series
© 2022 Koninklijke Philips N.V. (KPNV). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV.
Data on file - OHC-2019-10587

