A comparison of the effects of scaling and root planing, plus twice-daily toothbrushing, in a stage I/II periodontitis population
Salus Research, Inc. Indiana, USA Study completed in 2020
Objective
The objective of this study was to compare the effects of use of home oral hygiene with either a manual toothbrush or a Philips Sonicare powered toothbrush, on plaque and periodontal inflammation, in stage I/II periodontitis patients, following scaling and root planing, up to six months.
Methodology
This was an IRB-approved, randomized, parallel, single-blind clinical trial conducted in the United States. All subjects provided informed consent. Eligible subjects were non-smokers (>10 years), aged 18-75 years, with stage I/II periodontitis, confirmed by X-ray. Subjects with uncontrolled diabetes, or stage III or greater periodontal disease, were not eligible. Those enrolled on study received in-office scaling and root planing (SRP) within 4 weeks of the intake visit.
Thereafter, subjects were randomized to a home oral-hygiene regimen of either: twice-daily manual toothbrush (MTB) use (Colgate Classic Adult), or a Philips Sonicare DiamondClean Smart (DCS), used in Gum Health mode at high intensity, with Premium Gum Care brush head. Randomization was balanced for gender and periodontitis stage. The use of additional oral hygiene aids was prohibited over the study period. Subjects returned to clinic every four weeks for safety and efficacy evaluation, up to Month 6. A new brush head was provided for all participants every eight weeks.
The following efficacy parameters were assessed: Bleeding on Probing (BOP), Modified Plaque Index (MPI), Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL). Safety was evaluated via subject diary report and intraoral exam. In the event of accelerated or worsening oral health status that was felt to put the subject at undue risk, the principal investigator was able to exit subjects from study, as needed. (ClinicalTrials.gov Identifier: NCT04254770.)
Note, as a result of stay-at-home orders due to the COVID outbreak in the United States, in-clinic visits were suspended for all subjects at Weeks 8 and 12. Therefore, no efficacy outcomes are available for these timepoints. At these intervals, safety was conducted by interview and review of each subject’s diary card.
Results
There were 336 enrolled subjects. Of these, 163 were randomized to DCS, and 165 to MTB, with 149 DCS, and 150 MTB subjects who completed the study. The mean(SD) age of all randomized subjects was 44.1(13.2) years, with 229 females (105 with stage I periodontitis, 124 with stage II), and 99 males (42 with stage I, 57 with stage II).
Over the six-month study period, 33 subjects experienced at least one adverse event. None of the reported events were serious, or possibly related or related to study products. There were no reported unanticipated adverse device effects.
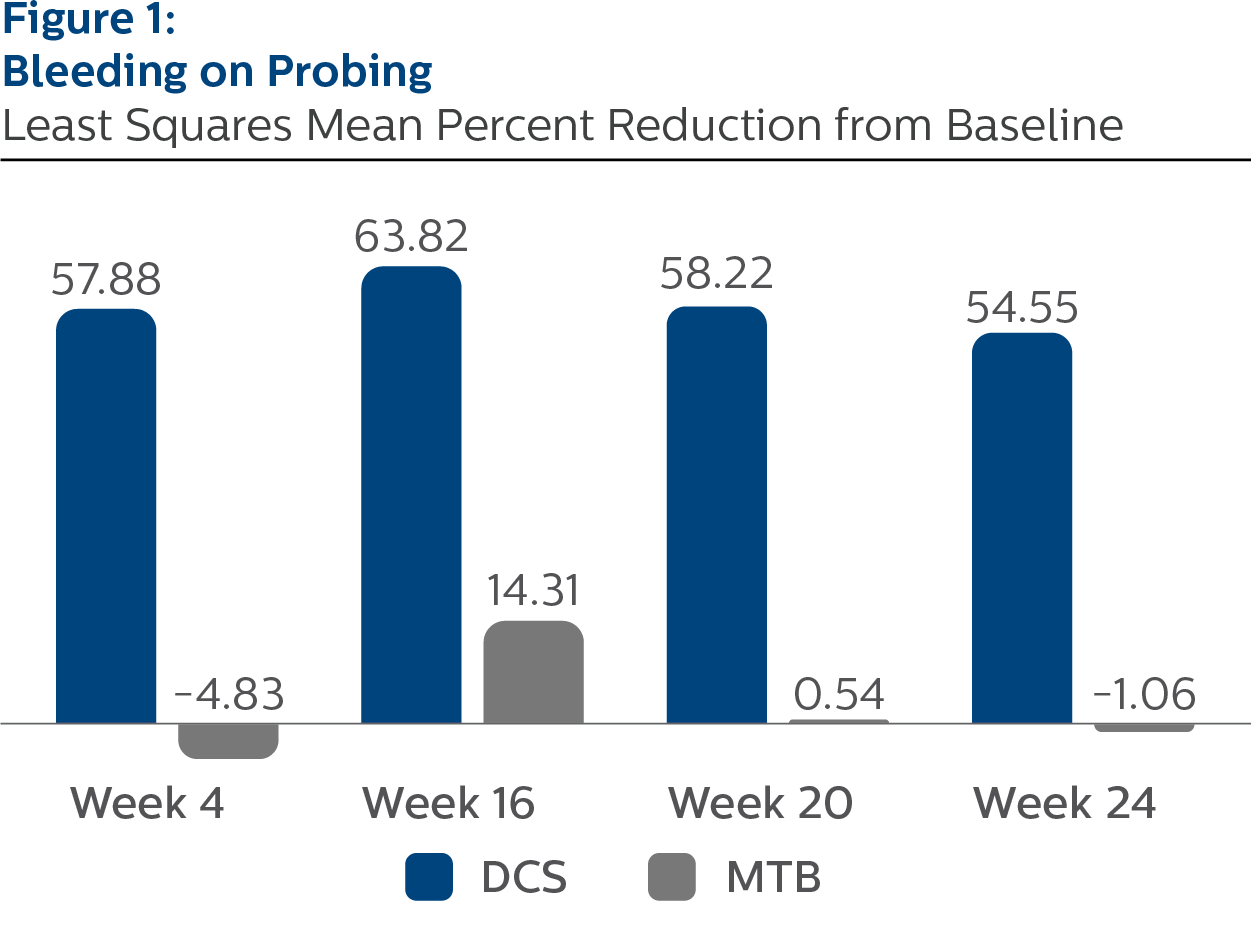
Bleeding on Probing
Baseline Values and LS Mean (SE) Percent Reduction from Baseline
| | DCS | MTB | p-value |
| Baselinea | 0.44 (0.01) | 0.43 (0.01) | 0.3974 |
| Percent Reduction from Baseline | | | |
| Week 4a | 57.88 (2.63) | -4.83 (2.62) | <.0001 |
| Week 16a | 63.82 (2.20) | 14.31 (2.21) | <.0001 |
| Week 20a | 58.22 (2.78) | 0.54 (2.84) | <.0001 |
| Week 24a | 54.55 (2.53) | -1.06 (2.52) | <.0001 |
a: this visit occurred up to 4 weeks prior to SRP b: this visit occurred 4 weeks after SRP
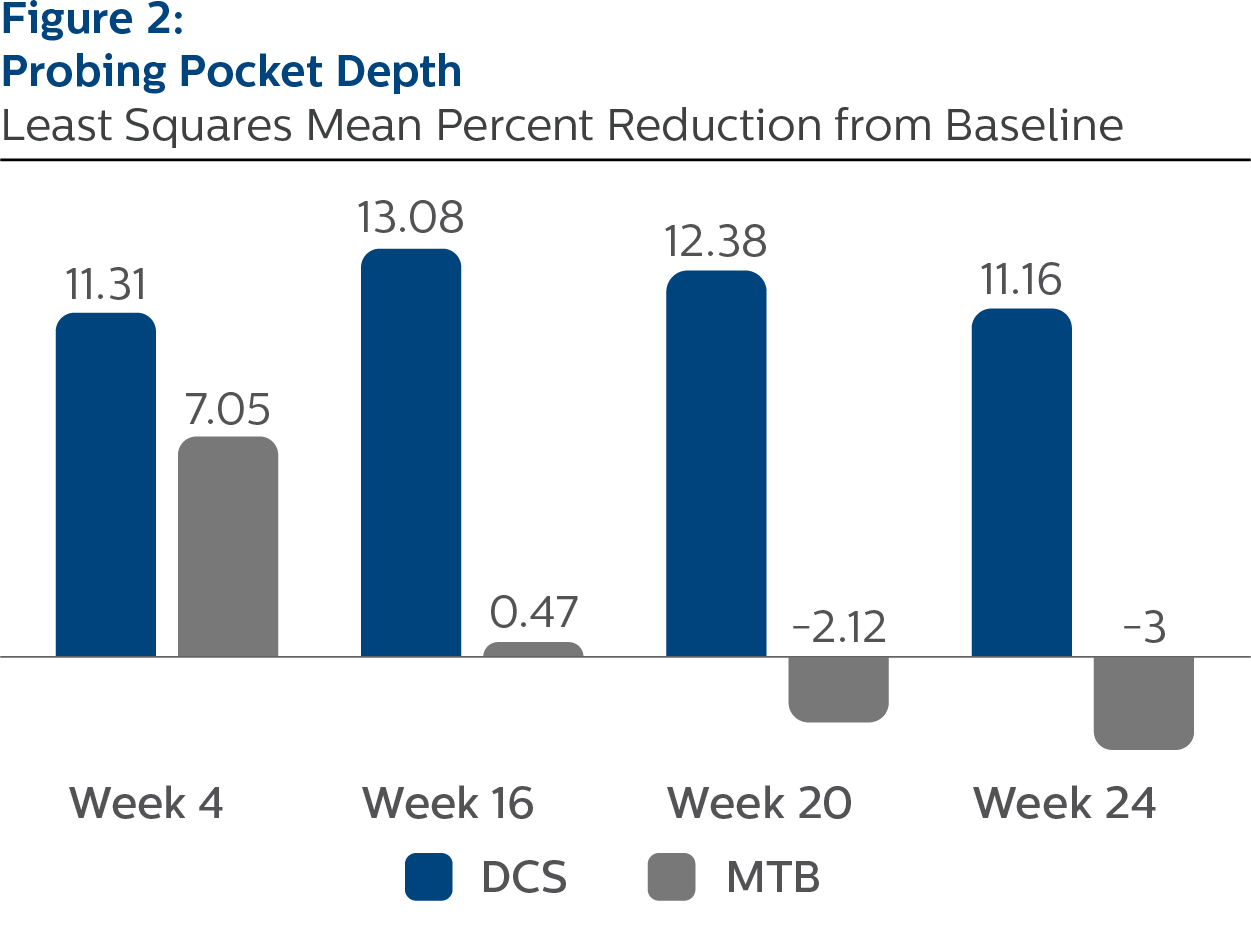
Probing Pocket Depth, All Sites
Baseline Values and LS Mean (SE) Percent Reduction from Baseline
| | DCS | MTB | p-value |
| Baseline | 1.65 (0.02) | 1.67 (0.02) | 0.2727 |
| Percent Reduction from Baseline | | | |
| Week 4 | 11.31 (0.35) | 7.05 (0.35) | <.0001 |
| Week 16 | 13.08 (0.37) | 0.47 (0.38) | <.0001 |
| Week 20 | 12.38 (0.43) | -2.12 (0.44) | <.0001 |
| Week 24 | 11.16 (0.39) | -3.00 (0.38) | <.0001 |
Clinical Attachment Level, All Sites
Baseline Values and LS Mean (SE) Percent Reduction from Baseline
| | DCS | MTB | p-value |
| Baseline | 1.15 (0.01) | 1.15 (0.01) | 0.8366 |
| Percent Reduction from Baseline | | | |
| Week 4 | 2.76 (0.26) | 1.88 (0.25) | 0.0148 |
| Week 16 | 13.08 (0.37) | 0.47 (0.38) | <.0001 |
| Week 20 | 3.36 (0.22) | 1.06 (0.22) | <.0001 |
| Week 24 | 3.13 (0.21) | 0.91 (0.21) | <.0001 |
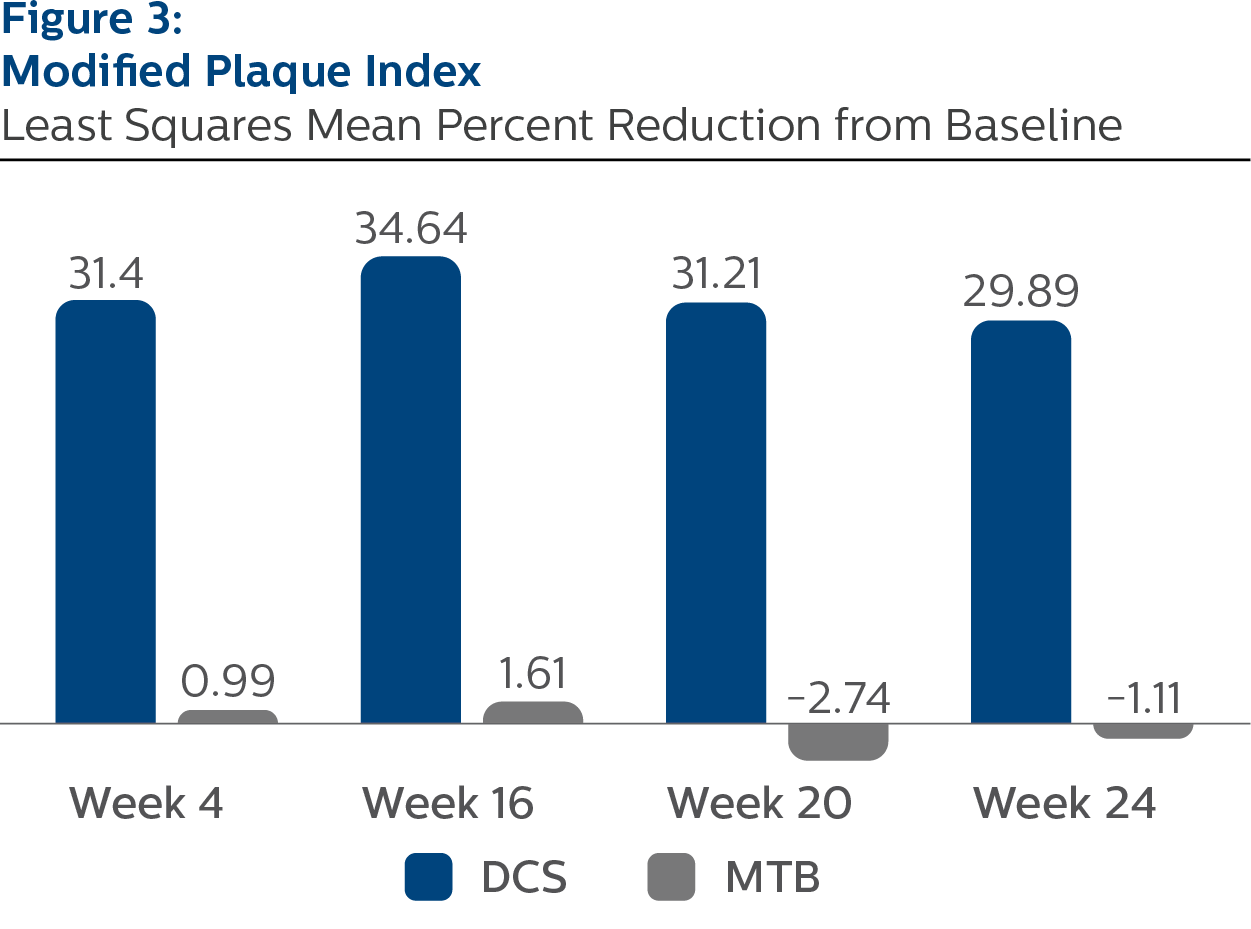
Modified Plaque Index
Baseline Values and LS Mean (SE) Percent Reduction from Baseline
| | DCS | MTB | p-value |
| Baseline | 2.84 (0.03) | 2.83 (0.03) | 0.8059 |
| Percent Reduction from Baseline | | | |
| Week 4 | 31.40 (1.04) | 0.99 (1.04) | <.0001 |
| Week 16 | 34.64 (1.09) | 1.61 (1.10) | <.0001 |
| Week 20 | 31.21 (1.04) | -2.74 (1.06) | <.0001 |
| Week 24 | 29.89 (0.90) | -1.11 (0.89) | <.0001 |
Conclusions
Following scaling and root planing, twice-daily home use of the Philips Sonicare DiamondClean Smart with Premium Gum Care brush head was statistically significantly more effective in reducing plaque, and symptoms of periodontal inflammation including bleeding and pocket depth, compared to use of a manual toothbrush. Both toothbrushes were safe on teeth and oral tissues.
© 2021 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV.
Data on file - OHC_10606
