Comparison of gingivitis and plaque reduction by Philips Sonicare and a manual toothbrush
Starke M, Mwatha A, Ward M, Argosino K, Jenkins W, Milleman J, Milleman K Salus Research, Ft. Wayne IN, USA

Objective
To evaluate the ability of the Philips Sonicare FlexCare toothbrush with an InterCare brush head to reduce gingivitis, gingival bleeding and plaque versus a manual toothbrush, following two and four weeks of product use.
Methodology
One hundred forty-eight adults (101 females; 47 males) with mild to moderate gingivitis, aged 18-65 years (mean age, 42.5 years) participated in a single-blind, randomized, parallel-design IRB-approved clinical study.
Eligible subjects were routine manual toothbrush users with a minimum Modified Plaque Index of ≥1.8 following three to six hours of plaque accumulation, and a Gingival Bleeding Index of ≥1 on at least 20 sites. Eligible subjects were randomized to either Philips Sonicare FlexCare toothbrush with an InterCare brush head (SFC) or ADA-reference manual toothbrush (MTB) for home use. Subjects were instructed to brush twice daily for a four-week period.
Efficacy and safety evaluations occurred at Weeks 2 and 4, in which gingivitis, bleeding and plaque levels were reassessed. Compliance was tracked at each follow-up visit by subject diary review. Safety was assessed by intraoral exam and subject report.
Results
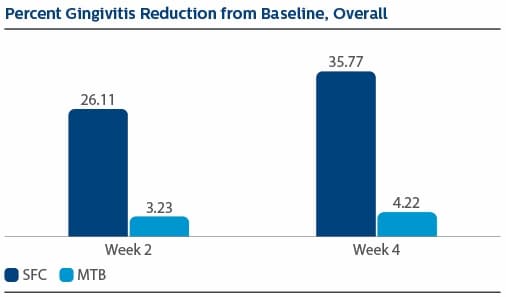
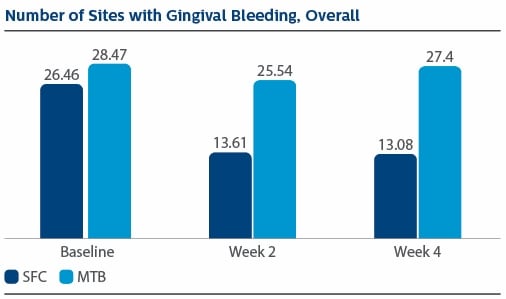
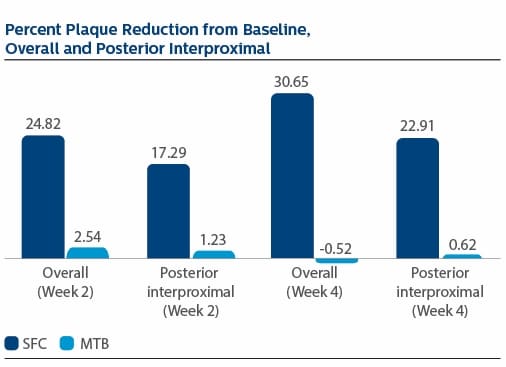
Modified Gingival Index (MGI) Following two weeks of product use, the LS Mean(SE) percent reduction in MGI was 26.11%(1.79) for SFC, and 3.23(1.79) for MTB, p-value <0.0001. The percent reduction in MGI at Week 4 was 35.77%(2.19) for SFC, and 4.22%(2.19) for MTB, p-value <0.0001. Following two weeks of product use, the LS Mean(SE) overall number of bleeding sites was 13.61(0.80) for SFC, and 25.54(0.80) for MTB, p-value <0.0001. The number of bleeding sites at Week 4 was 13.08(0.92) for SFC, and 27.40(0.92) for MTB, p-value <0.0001. Following two weeks of product use, the LS Mean(SE) percent reduction in MPI was 24.82%(1.40) for SFC, and 2.54(1.40) for MTB, p-value<0.0001. The percent reduction in MPI at Week 4 was 30.65%(1.49) for SFC, and -0.52%(1.49) for MTB, p-value <0.0001.
Gingival Bleeding Index
Modified Plaque Index
There were no adverse events reported.
Conclusion
In a population of subjects with mild to moderate gingivitis, the Philips Sonicare FlexCare toothbrush with InterCare brush head was significantly superior to a manual toothbrush in reducing gingivitis, gingival bleeding and plaque folllowing two and four weeks of home use.
Both products were safe for use, including for subjects with functional and cosmetic restorations.
© 2019 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV and/or Philips Oral Healthcare, LLC.
Data on file - MAH-12-066

