In-vitro biofilm removal from human enamel using a Philips Sonicare Power Flosser

Objective
Quantify removal of in-vitro dental biofilm using the Philips Sonicare Power Flosser oral irrigator.
Methodology
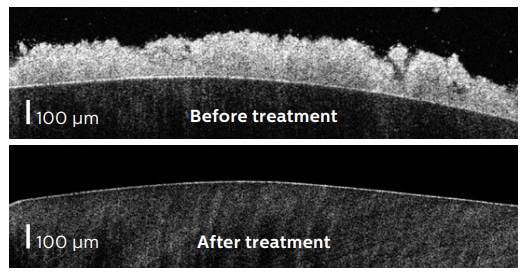
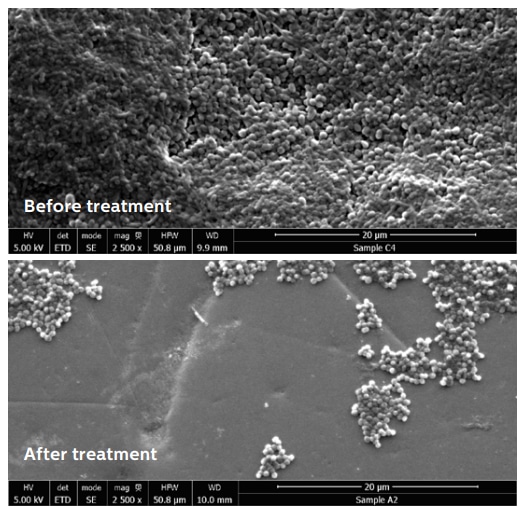
Dental biofilms were grown from pooled human saliva on human enamel disks for 4 days, according to an established academic model*. The biofilms (n=6) were treated with the Philips Sonicare Power Flosser for 3 seconds using the Quad Stream nozzle. To quantify the number of bacteria before treatment, the biofilm volume was measured using optical coherence tomography (OCT) and the bacterial cell density was determined from untreated control samples (n=6) using confocal laser scanning microscopy (CLSM). After treatment the number of remaining bacteria were counted using CLSM. Additionally scanning electron microscope (SEM) images were recorded.
Results
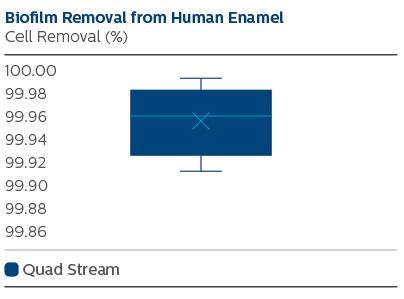
While before treatment 0.2 mm thick dense biofilms were present, after treatment only scattered groups of bacteria remained. Quantitative analysis showed 99.96% removal for the Quad Stream nozzle.
Conclusions
The Philips Sonicare Power Flosser oral irrigator with Quad Stream nozzle removes over 99.9% of the bacteria in this established laboratory model of dental biofilm.
* Exterkate RA, Crielaard W, Ten Cate JM. Di erent response to amine uoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 2010;44(4):372-9.
© 2022 Koninklijke Philips N.V. (KPNV). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of
KPNV. Data on file - PR-TN 2019/00361

