Whitening
In-vivo study
A two-phase, three-month clinical evaluation comparing two chairside tooth bleaching treatments, with tooth shade maintenance by powered or manual toothbrushing
Lee SS, Kwon SR. Ward M, Jenkins W, Souza S, Li Y. A 3 months clinical evaluation comparing two professional bleaching systems of 25% and 40% hydrogen peroxide and extended treatment outcome using a power versus a manual toothbrush, J Esthet Restor Dent. 2019 Mar;31(2):124-131. Center for Dental Research, Loma Linda University, USA

Objective
For phase one, objectives included comparisons of the effects of chair-side tooth bleaching on tooth color and shade immediately, seven days and 30 days following treatment. For phase two, maintenance of tooth color and shade was compared between a powered toothbrush and a manual toothbrush, Day 30 to Day 90. Tooth sensitivity and safety was monitored throughout the study.
Methodology
This was an IRB-approved, randomized, parallel, two-phase clinical trial. Eligible subjects were generally healthy adults, aged 18-75 years, presenting with a VITA Classical shade (VCS) of A3 or darker on at least four maxillary anterior teeth. In Phase I, subjects were randomized to receive chairside tooth bleaching with either Philips Zoom WhiteSpeed (PZW), 25% H2O2 and LED acceleration), or Ultradent Opalescence Boost PF ((UOB), 40% H2O2). Both the subject and the Examiners were blinded to the assigned treatment. Tooth color and tooth shade were assessed using VITA EasyShade (VES) for ΔE, VCS, and VITA BleachedGuide (VBG), with evaluations at pre-treatment, immediately, Day 7 and Day 30 following tooth-bleaching. Safety was characterized by subject report of sensitivity, oral examination and subject use of sensitivity-reducing agents (Relief ACP for PZW subjects, or UltraEZ for UOB subjects) applied per manufacturer’s instructions. All subjects used a standard manual toothbrush during study Phase I. On Day 30, approximately equal numbers of subjects from the PZW and UOB treatment groups were then randomized to long-term tooth-bleaching maintenance with either a Philips Sonicare DiamondClean (SDC) powered toothbrush, or a manual toothbrush (MTB). All subjects were provided a standard dentifrice. Subjects returned to clinic at Day 90 for final tooth shade and color assessments.
Results
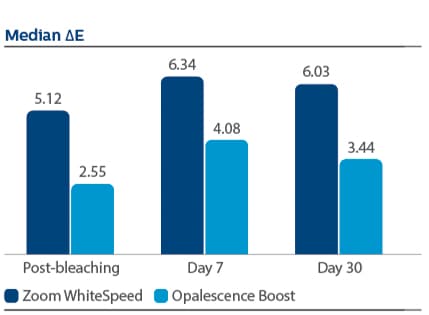
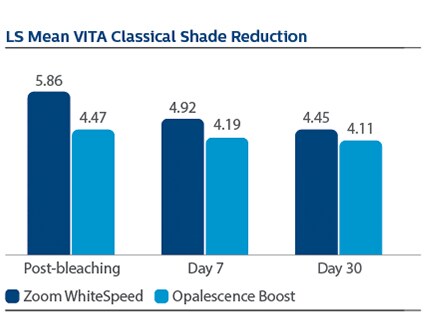
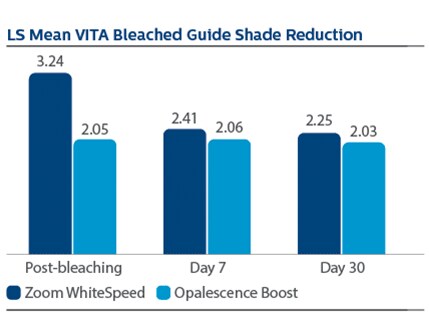
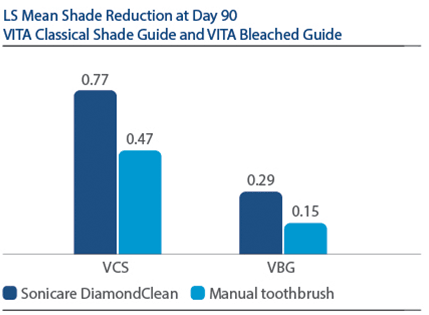
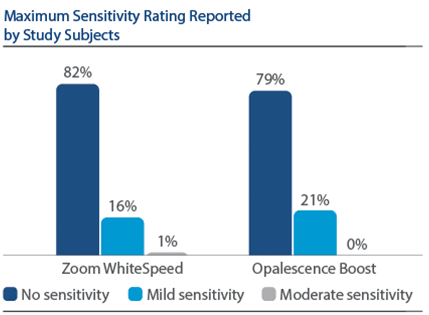
Demographics Of 394 subjects screened, 136 were enrolled and randomized in Phase I, 67 to PZW and 69 to UOB (mean age, 50 years). Of these, 134 were randomized in Phase II, 67 to SDC and 67 to MTB. One hundred thirty-three subjects completed the study. Phase I efficacy For the primary endpoint, DE at Day 7, a significantly larger reduction was observed for PZW than UOB, with Kruskal-Wallis median DE values of 6.34 and 4.08, respectively, p-value = 0.0059. Significant differences in tooth shade were also observed at Day 7 per VCS, with LS Mean (SE) reductions of 4.92 (0.20) for PZW, and 4.19 (0.20) for UOB, p-value = 0.0106. Significant differences in tooth shade at Day 7 were also observed per VBG, with LS Mean (SE) reductions of 2.41 (0.13) for PZW, and 2.06 (0.12) for UOB, p-value = 0.0489. Phase II efficacy On Day 90, the SDC was statistically superior to MTB in maintaining shade per VCS, with LS Mean (SE) reduction of 0.77 (0.22) for SDC and 0.47 (0.22) for MTB, p-value = 0.0001. For VBG at Day 90, the LS Mean (SE) reduction was 0.29 (0.12) for SDC and 0.15 (0.12) for MTB, p-value = 0.0108. No tooth color differences were observed per DE. Safety The percentage of subjects who reported “no sensitivity” immediately post-bleaching was 98.5% for PZW, and 98.6% for UOB. At Day 7, these values were 82.1% for PZW, and 79.4% for UOB. Of those who did experience sensitivity, one subject rated sensitivity as “moderate.” All other reports were characterized as “mild.” There was a total of 41 adverse events reported among 34 subjects. In general, these events were associated with sensitivity. Subject use of post-bleaching sensitivity gel (Relief ACP and UltraEZ) was low. Four subjects (two per treatment group) used the products at Day 1 post-bleaching, and one subject used the product on Day 2. There are no other reports of use thereafter.
Conclusions
At Day 7 following tooth bleaching, Philips Zoom WhiteSpeed showed statistically greater change in overall tooth color and shade than Ultradent Opalescence Boost PF. At Day 90 following tooth bleaching, Philips Sonicare DiamondClean powered toothbrush maintained tooth shade significantly better than a manual toothbrush. Both chairside tooth bleaching products and the toothbrushing regimens are safe for use.
© 2022 Koninklijke Philips N.V. (KPNV). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV. Data on file - DRC 1718
1 Jan, 2019 by Philips
Reading time: 4-5 minutes
Share on social media
Contacts

Sarah Berryhill
Clinical Marketing Team
You are now exiting the Philips United States (US) site and entering the Philips global site. This content is intended for a global audience. It may not apply to the US and should not be interpreted as meeting US standards, executive orders or regulations.
Continue Assets