Oral malodor
In-vivo study
A randomized, parallel study to assess the effect of three tongue cleaning modalities on oral malodor
Li Y, Lee S, Stephens J, Zhang W, Suprono M, Mirza F, Ward M, Mwatha T Center for Dental Research, Loma Linda University, USA
(J Clin Dent 2019;30(Spec Iss A)A30–38)

Objectives
The primary objective of this study was to assess the extent to which Philips Sonicare TongueCare+ improves breath odor compared to manual tongue brushing alone, and to Listerine Cool Mint Rinse alone, based on organoleptic assessment. The secondary objectives of the study included safety evaluation, and the effects of the study products on hydrogen sulfide levels in exhaled breath and tongue microbes (aerobes, anaerobes).
Methodology
One hundred sixty-eight healthy adults meeting study requirements were enrolled in this IRB-approved, parallel, examiner-blinded, three-arm clinical trial. There were 91 female and 77 male participants (mean age, 38.9 years) with 56 subjects per treatment group. Eligible subjects were non-smokers aged 18–70 years with a minimum organoleptic (OL) score of 2.7–4.5 following 12–18 hours of oral hygiene abstention. The OL score was an average, computed following breath assessment by three calibrated, blinded organoleptic assessors. Eligible participants agreed to comply with study procedures related to oral hygiene, and food/drink restrictions. The breath hygiene study groups were: There were two study visits (Day 1 and Day 8) with assessments occurring at each visit at the following intervals: upon arrival, immediately, and four and eight hours following breath hygiene product use. Organoleptic assessments, as well as microbial at H2S samples were performed at each of these time points. In addition to the assigned breath hygiene regimen, all subjects used a standardized home toothbrushing regimen (Philips Sonicare power toothbrush and Crest Cool Mint Gel dentifrice) twice daily, during the seven-day home-use period.
Results
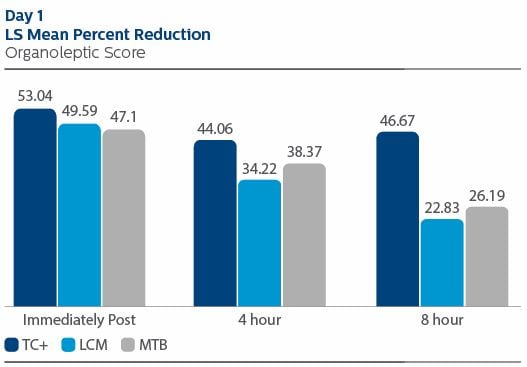
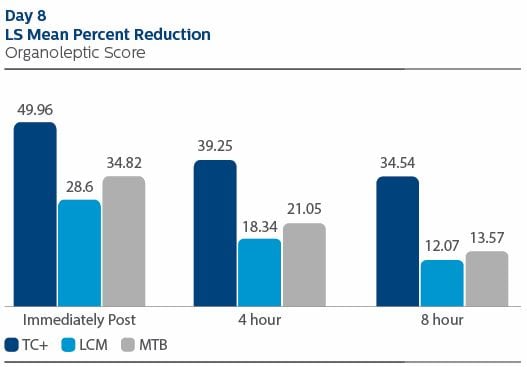
Organoleptic evaluation On Day 1 immediately post treatment, the LS Mean (95% CI) percent reduction from pre-treatment for OL was 53.04% (49.37%, 56.71%) for TC+, 49.59% (45.90%, 53.28%) for LCM and 47.1% (43.40%, 50.79%) for MTB. The pair-wise comparison between TC+ and MTB was statistically significant (p-value = 0.0330). At four hours post-treatment, the percent reduction in OL was 44.06% (39.89%, 48.23%) for TC+, 34.22% (30.03%, 38.41%) for LCM, and 38.37% (34.16%, 42.57%) for MTB. The pair-wise comparison between TC+ and LCM was statistically significant (p-value = 0.0062). At eight hours post-treatment, the percent reduction in OL was 46.67% (42.18%, 51.57%) for TC+, 22.83% (18.31%, 27.35%) for LCM and 26.19% (21.66%, 30.72%) for MTB. The differences between TC+ and MTB, and TC+ and LCM, were significant (p-value < 0.0001, for each pair-wise comparison). For OL outcomes on Day 8, the TC+ treatment group exhibited the lowest value throughout the study visit. Statistically significant differences were observed between TC+ and LCM, and TC+ and MTB, at each time point: immediately, four hours and eight hours following product use. Values are included below. The reported safety events were mild in severity and were unrelated to test product use.
Conclusions
The Philips Sonicare TongueCare+ breath hygiene regimen was effective at improving oral malodor. Improvements, based on organoleptic assessment, were evident immediately following, and up to eight hours following product use. The Philips Sonicare TongueCare+ breath hygiene regimen improved oral malodor significantly better than either Listerine Cool Mint Antiseptic Rinse, or tongue brushing with an ADA-reference manual toothbrush, up to eight hours following product use. *For brevity, the outcomes for the secondary efficacy endpoints, H2S and microbes, are not reported here. Please refer to the full publication for these details. © 2019 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV and/or Philips Oral Healthcare, LLC. Data on file - MAH-0172
1 Jan, 2019 by Philips
Reading time: 4-5 minutes
Share on social media
Contacts

Sarah Berryhill
Clinical Marketing Team
You are now exiting the Philips United States (US) site and entering the Philips global site. This content is intended for a global audience. It may not apply to the US and should not be interpreted as meeting US standards, executive orders or regulations.
Continue Assets