Gingival health
In-vivo study
A comparison of Philips Sonicare Cordless Power Flosser and a manual toothbrush with three other interdental cleaning modalities on gingivitis and plaque after a six-week period of home use
Starke M, Nelson M, Foster J, Ward M, Milleman K, Milleman J Salus Research Inc., Fort Wayne IN, USA

Objective
To evaluate the effect of four different cleaning modalities on gingivitis, gingival bleeding and surface plaque following six weeks of home use. The different cleaning modalities included the manual toothbrush (MTB) alone, the MTB used with the Philips Sonicare Cordless Power Flosser, the MTB used with an interdental brush and the MTB used with string floss.
Methodology
Three hundred seventy-two adults (279 females, 93 males) aged 18-65 years (mean age: 43.2 years), participated in a 4-arm, 3-visit, single-blind, randomized, parallel-design, IRB-approved clinical study conducted at a single center. Eligible subjects were regular MTB users with moderate to severe gingivitis that included an average Rustogi Modified Navy Plaque Index (RMNPI) score of ≥0.6 following three to six hours of plaque accumulation and a Gingival Bleeding Index (GBI) score of ≥1 on at least 50 gingival sites. Subject scores for RMNPI, GBI and the Modified Gingival Index (MGI) at baseline were similar. Eligible subjects were randomized to one of four treatment arms: use of the Colgate® Classic MTB alone (CTB group), use of the MTB and the Philips Sonicare Cordless Power Flosser used in the clean mode with high intensity along with the QuadStream nozzle (SPF group), use of the MTB and a TePe® interdental brush (IDB group) or use of the MTB and Reach® unflavored waxed string floss (FLS group). All subjects were provided with Crest® Cool Mint toothpaste to use for the duration of the study and were asked to refrain from any other at-home oral hygiene modalities. Subjects were instructed to brush at home with the MTB for one minute twice daily for a six-week period. For those groups also assigned to an additional interdental cleaning modality other than the MTB alone, subjects were instructed to use the additional modality once daily in the evening per provided instructions. Efficacy and safety evaluations occurred at Weeks 2 and 6, in which surface plaque, gingivitis, and bleeding levels were reassessed. Compliance was tracked by subject diary review. Safety was assessed by subject report and oral examination.
Results
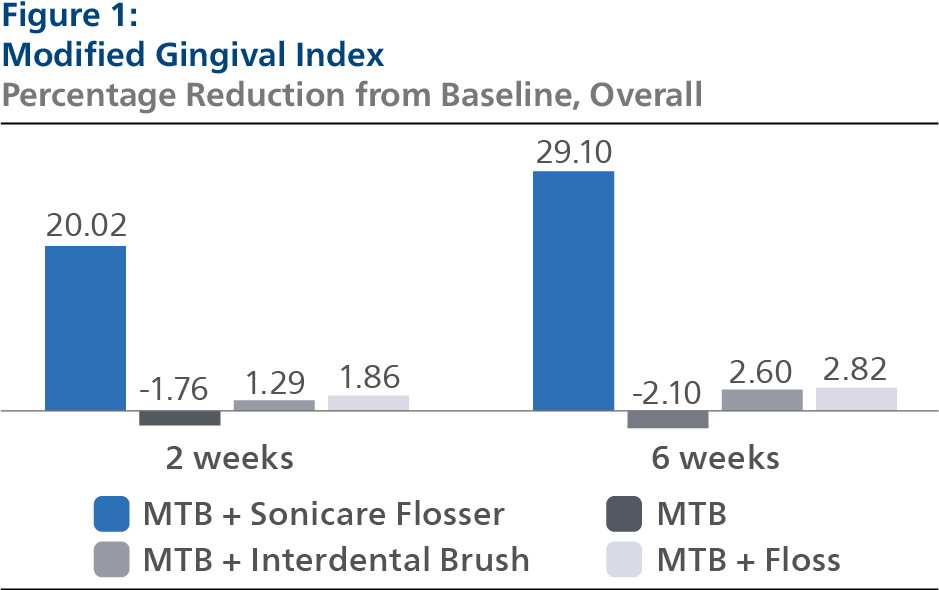
Modified Gingival Index (MGI) At Baseline, the MGI Least Squares (LS) mean and 95% Confidence Interval (CI) outcomes were 2.70 (2.66, 2.75) for SPF, 2.74 (2.69, 2.78) for CTB, 2.71 (2.67, 2.75) for IDB and 2.74 (2.69, 2.78) for FLS, p-value = 0.5934. Following two weeks of product use, the MGI LS mean and 95% CI outcomes were 2.18 (2.15, 2.21) for SPF, 2.76 (2.73, 2.79) for CTB, 2.68 (2.65, 2.71) for IDB and 2.67 (2.64, 2.70) for FLS, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 20.02% (18.85%, 21.19%) for SPF, -1.76% (-2.93%, -0.58%) for CTB, 1.29% (0.10%, 2.47%) for IDB and 1.86% (0.68%, 3.05%) for FLS, p-value < 0.0001. Following six weeks of product use, the MGI LS mean and 95% CI outcomes were 1.94 (1.89, 1.98) for SPF, 2.77 (2.72, 2.82) for CTB, 2.64 (2.60, 2.69) for IDB and 2.64 (2.59, 2.69) for FLS, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 29.10% (27.32%, 30.88%) for SPF, -2.10% (-3.85%, -0.36%) for CTB, 2.60% (0.83%, 4.38%) for IDB and 2.82% (1.07%, 4.58%) for FLS, p-value < 0.0001. Pairwise comparisons of the MGI for the four groups indicated that there was a statistically significant difference (p<0.0001) between the SPF group and each of the other treatment groups at both the 2- and 6-week timepoints.
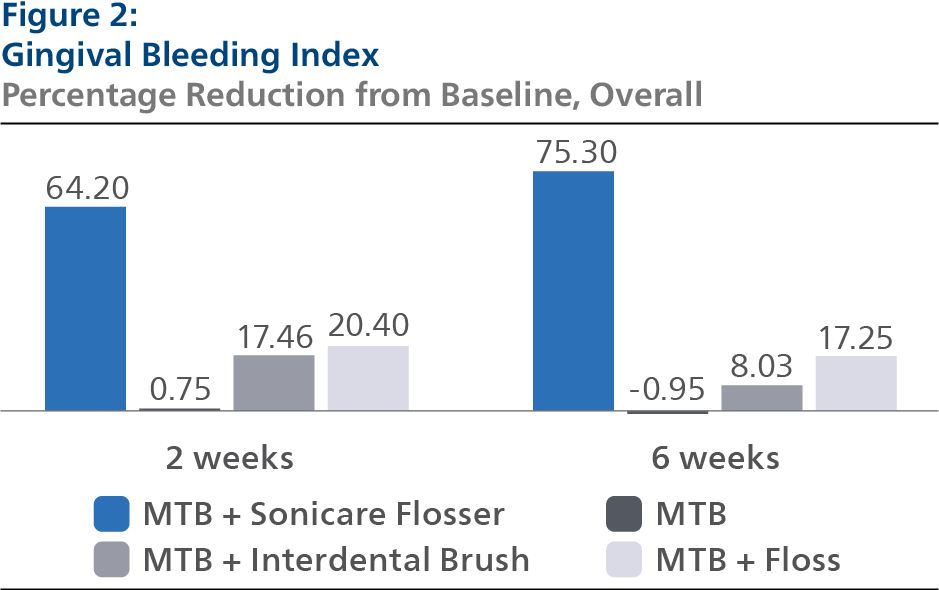
Gingival Bleeding Index (GBI) At Baseline, the GBI LS mean and 95% Confidence Interval (CI) outcomes were 0.46 (0.43, 0.50) for SPF, 0.48 (0.45, 0.52) for CTB, 0.46 (0.43, 0.49) for IDB and 0.47 (0.44, 0.50) for FLS, p-value = 0.7165. Following two weeks of product use, the GBI LS mean and 95% CI outcomes were 0.17 (0.15, 0.19) for SPF, 0.46 (0.44, 0.48) for CTB, 0.38 (0.36, 0.40) for IDB and 0.37 (0.35, 0.39) for FLS, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 64.20% (60.26%, 68.13%) for SPF, 0.75% (-3.21%, 4.72%) for CTB, 17.46% (13.48%, 21.44%) for IDB and 20.40% (16.42%, 24.38%) for FLS, p-value < 0.0001. Following six weeks of product use, the GBI LS mean and 95% CI outcomes were 0.13 (0.10, 0.15) for SPF, 0.47 (0.44, 0.50) for CTB, 0.43 (0.40, 0.46) for IDB and 0.38 (0.36, 0.41) for FLS, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 75.30% (70.31%, 80.30%) for SPF, -0.95% (-5.86%, 3.97%) for CTB, 8.03% (3.04%, 13.02%) for IDB and 17.25% (12.32%, 22.19%) for FLS, p-value < 0.0001. Pairwise comparisons of the GBI for the four groups indicated that there was a statistically significant difference (p<0.0001) between the SPF group and each of the other treatment groups at both the 2- and 6-week timepoint.
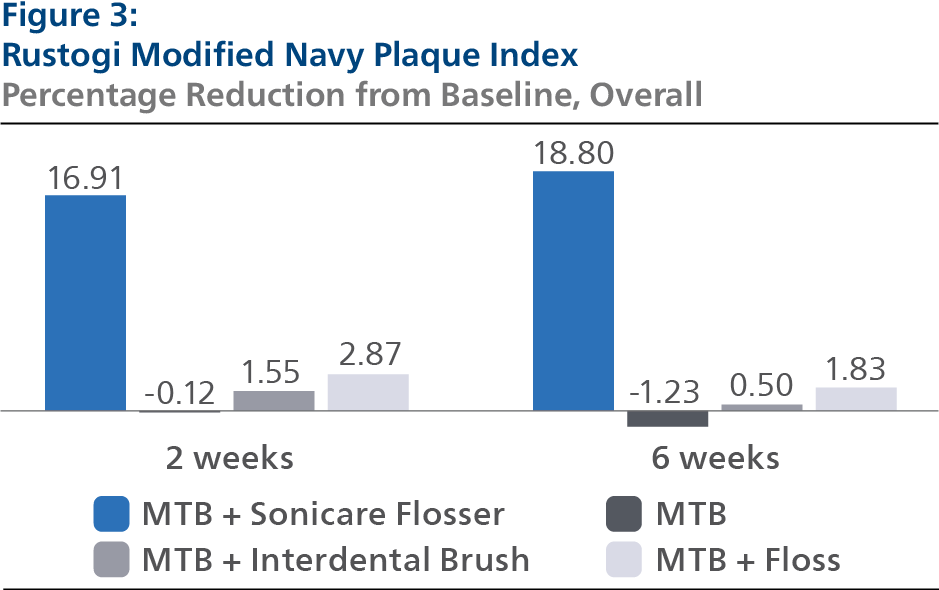
Rustogi Modified Navy Plaque Index (RMNPI) At Baseline, the RMNPI LS mean and 95% Confidence Interval (CI) outcomes were 0.84 (0.83, 0.85) for SPF, 0.84 (0.83, 0.86) for CTB, 0.83 (0.81, 0.84) for IDB and 0.84 (0.83, 0.86) for FLS, p-value = 0.3678. Following two weeks of product use, the RMNPI LS mean and 95% CI outcomes were 0.69 (0.68, 0.70) for SPF, 0.84 (0.83, 0.85) for CTB, 0.82 (0.81, 0.83) for IDB and 0.81 (0.80, 0.82) for FLS, p-value< 0.0001. Expressed as percent reduction from Baseline overall, this was 16.91% (15.75%, 18.06%) for SPF, -0.12% (-1.28%, 1.04%) for CTB, 1.55% (0.38%, 2.72%) for IDB and 2.87% (1.70%, 4.04%) for FLS, p-value < 0.0001. Following six weeks of product use, the RMNPI LS mean and 95% CI outcomes were 0.68 (0.67, 0.69) for SPF, 0.85 (0.84, 0.86) for CTB, 0.83 (0.82, 0.84) for IDB and 0.82 (0.81, 0.83) for FLS, p-value < 0.0001. Expressed as percent reduction from Baseline overall, this was 18.80% (17.54%, 20.06%) for SPF, -1.23% (-2.47%, 0.01%) for CTB, 0.50% (-0.77%, 1.76%) for IDB and 1.83% (0.59%, 3.08%) for FLS, p-value < 0.0001. Pairwise comparisons of the RMNPI forthe four groups indicated that there was a statistically significant difference (p < 0.0001) between the SPF group and each of the other treatment groups at both the 2- and 6-week timepoints.
Gingival Health Using the American Academy of Periodontology / European Federation of Periodontology guidelines where ‘not healthy’ is defined as having ≥ 10% bleeding sites in the whole mouth, all study subjects were ‘not healthy’ at baseline. After two weeks of home use, 16 (17%) subjects in the SPF group converted to a ‘healthy’ status with <10% bleeding sites in the whole mouth. After six weeks of home use, 44 (50%) subjects in the SPF group converted to a ‘healthy” status. No other subjects in any of the other groups at either timepoint converted to ‘healthy” status. Safety There were three adverse events reported in the study, one each in the SPF, CTB and FLS groups. All adverse events were rated as mild and none were classified as related to the study or study products.
Conclusions
An interdental cleaning regimen combining a manual toothbrush and the Philips Sonicare Cordless Power Flosser used in the clean mode with high intensity along with the QuadStream nozzle was statistically superior to a manual toothbrush alone, a manual toothbrush used with an interdental brush and a manual toothbrush used with string floss in reducing gingival inflammation, gingival bleeding and surface plaque following two- and six-week periods of twice-daily home use. All products tested are safe for home use.
Data on file (2022) © 2022 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of Philips Oral Healthcare, LLC. SONICARE and the Sonicare logo are trademarks of Philips Oral Healthcare, LLC. Data on file - OHC-11265
1 Jan, 2022 by Philips
Reading time: 4-5 minutes
Share on social media
Contacts

Sarah Berryhill
Clinical Marketing Team
You are now exiting the Philips United States (US) site and entering the Philips global site. This content is intended for a global audience. It may not apply to the US and should not be interpreted as meeting US standards, executive orders or regulations.
Continue Assets