Gingival health
In-vivo study
Comparison of plaque and gingivitis reduction by Philips Sonicare DiamondClean Smart toothbrush with Premium Gum Care brush head in Gum Health mode or Clean mode and a manual toothbrush
Milleman J, Milleman K, Olson M, Ou S, Souza S, Starke M, Ward M Salus Research, Ft. Wayne IN, USA (J Clin Dent 2019;30(Spec Iss A)A16–23)

Objective
To compare the effects of a Philips Sonicare DiamondClean Smart toothbrush with a Premium Gum Care brush head and an ADA-reference manual toothbrush on plaque and gingivitis following two and six weeks of home use.
Methodology
One hundred eighty-eight adults (mean age 43.6 years; 137 female/51 male) completed this IRB-approved, single-center, three-arm, examiner-blind, parallel-design clinical trial. Eligible subjects were routine manual toothbrush users who were non- smokers, aged 18–65 with a minimum plaque score of ≥1.8 per Lobene and Soparker Modified Plaque Index (MPI) following 3–6 hours of plaque accumulation, and a Gingival Bleeding Index (GBI) of ≥1 on at least 20 sites. The treatment groups included the following: Philips Sonicare DiamondClean Smart toothbrush with a Premium Gum Care brush head used in Gum Health mode (DC-GH), the same powered toothbrush used in Clean mode (DC-C), and an ADA-reference manual toothbrush (MTB). The primary objective of this study was to compare the effects of twice daily use of DC-GH to MTB. All study products were used with a standard fluoride-containing dentifrice. The use of any other oral hygiene procedure was prohibited during the study period. Gingivitis, (Modified Gingival Index (MGI)), gingival bleeding (GBI), and MPI efficacy metrics were assessed at Baseline, and following two and six weeks of home use of the study products. Subjects presented to clinic for all visits with 3-6 hours of plaque accumulation. Safety was assessed by intraoral exam and per subject report.
Results
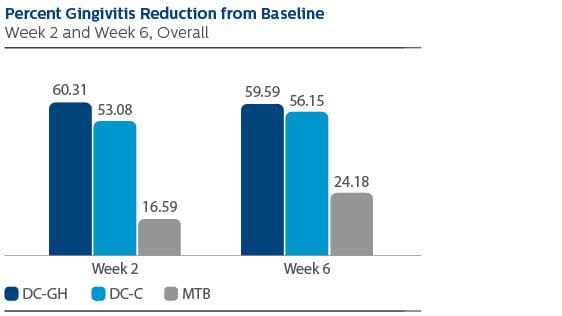
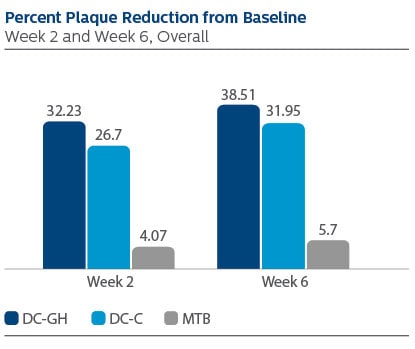
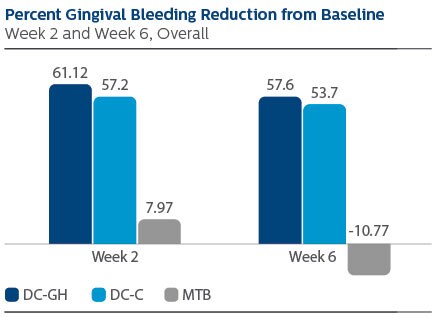
Modified Gingival Index (MGI) Following two weeks of product use, the LS Mean (95% Confidence Interval (CI)) percent reduction from Baseline was 60.31% (56.47%, 64.15%) for DC-GH, 53.08% (49.24%, 56.92%) for DC-C, and 16.59% (12.71%, 20.46%) for MTB. Following six weeks of product use, the percent reduction was 59.59% (55.54%, 63.64%) for DC-GH, 56.15% (52.11%, 60.20%) for DC-C, and 24.18% (20.09%, 28.27%) for MTB. Statistical superiority was observed for each power toothbrush group compared to the MTB group, p-value < 0.0001, at both Week 2 and Week 6. Gingival Bleeding Index (GBI) Following two weeks of product use, the LS Mean (95% CI) percent reduction from Baseline was 61.12% (53.71%, 68.53%) for DC-GH, 57.20% (49.79%, 64.61%) for DC-C and 7.97% (0.52%,15.41%) for MTB. Following six weeks of product use, the percent reduction was 57.60% (48.68%, 66.52%) for DC-GH, 53.70% (44.78%, 62.62%) for DC-C, and -10.77% (-19.73%, -1.81%) for MTB. Statistical superiority was observed for each power toothbrush group compared to the MTB group, p-value < 0.0001, at both Week 2 and Week 6. Modified Plaque Index (MPI) Following two weeks of product use, the LS Mean (95% CI) percent reduction from Baseline was 32.23% (29.43%, 35.03%) for DC-GH, 26.70% (23.90%, 29.51%) for DC-C, and 4.07% (1.25%, 6.90%) for MTB. Following six weeks of product use, the percent reduction was 38.51% (35.35%, 41.67%) for DC-GH, 31.95% (28.79%, 35.11%) for DC-C, and 5.70% (2.51%, 8.88%) for MTB. Statistical superiority was observed for each power toothbrush group compared to the MTB group, p-value < 0.0001, at both Week 2 and Week 6. Safety There were two adverse events reported, one of which was reported as mild, the other as moderate. Both events were deemed possibly related to the study product.
Conclusions
The use of a Philips Sonicare DiamondClean Smart toothbrush with Premium Gum Care brush head, used in either Gum Health mode or Clean mode, was statistically significantly superior to an ADA-reference manual toothbrush in reducing gingival inflammation, gingival bleeding and surface plaque following two and six weeks of home use. Both products were safe for home use.
© 2019 Koninklijke Philips N.V. (KPNV ). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV and/or Philips Oral Healthcare, LLC. Data on file - MAH-16-0188
1 Jan, 2019 by Philips
Reading time: 4-5 minutes
Share on social media
Contacts

Sarah Berryhill
Clinical Marketing Team
You are now exiting the Philips United States (US) site and entering the Philips global site. This content is intended for a global audience. It may not apply to the US and should not be interpreted as meeting US standards, executive orders or regulations.
Continue Assets