Comparison of the tooth shade reduction and color change effects of 6% hydrogen peroxide with and without Philips Zoom WhiteSpeed led acceleration followed by use of Philips Zoom NiteWhite 16% carbamide peroxide
Ontiveros J, Eldiwany MS, Arriaga DM, Fay RM, Gonzalez MD, Pereira Sanchez NA, Sly MM, Paravina R. Clinical efficacy & sensitivity on in-office tooth whitening with & without light treatment combined with at-home bleaching. J Cosmetic Dent. Winter 2019. Vol 34 (4): 70-79.

Objective
The primary objective of this study was to compare tooth shade reduction following application of 6% hydrogen peroxide with and without Philips Zoom WhiteSpeed LED acceleration, immediately and seven days following treatment.
Secondary objectives included an evaluation of safety, tooth color at all timepoints, and tooth shade at Day 14 post in-office bleaching, which included three-dose use of Philips Zoom NiteWhite 16% carbamide peroxide.
Methodology
This was a randomized, single-blind, split-mouth (opposing arch) design clinical trial. The IRB-approved study was conducted in generally healthy subjects, at least 18 years of age, who presented with a minimum of four of six anterior facial-maxillary teeth assessed as 2.5M2/3M2 per VITA BleachedGuide 3D Master Shade Guide. Subjects with intrinsic tooth staining (tetracycline exposure, fluorosis) or with visible supragingival calculus on anterior teeth, or with a contraindicating medical or dental condition, were excluded from the study. Enrolled subjects were fitted with a custom jig (one for each arch) for all CIE (ΔE) color measurements using the VITA EasyShade spectrophotometer. Tooth shade matching was performed using the VITA BleachedGuide 3D-Master (VBG) by calibrated examiners, according to ISO/TR 286422, under controlled lighting conditions, at a distance of approximately 25 cm. Shade change was expressed in terms of shade guide units (SGU), computed as the difference from Baseline in the absolute number of shade guide “steps”. Per ISO/TR 28642, color difference values above ΔE* = 2.7 were considered to represent a color mismatch beyond the 50:50% acceptability threshold (AT), and above ΔE* = 1.2 beyond the 50:50% perceptibility threshold (PT).
Study subjects were randomly assigned to opposing arch (maxillary versus mandibular arch) in-office tooth bleaching with 6% hydrogen peroxide (HP) with and without Philips Zoom WhiteSpeed LED acceleration. The facial surface of the anterior teeth in each arch were isolated and treated with 6% HP for 15 minutes, in four cycles. Efficacy (VBG, ΔE) of 6% HP with and without LED acceleration was assessed at Baseline, immediately following, and at Day 7 and Day 14 following in-office bleaching. At the Day 7 visit, all subjects were dispensed a take-home Philips Zoom NiteWhite 16% carbamide peroxide kit to be used over a three-dose period (three overnight applications). All study subjects used a soft-bristle manual toothbrush with Sensodyne True White dentifrice for the duration of the study.
Results
Of 82 subjects consented and enrolled, 77 subjects completed the study, (mean age: 49 years, 43 females, 34 males).
VITA BleachedGuide 3D Master, Shade Guide Units (SGU)
Immediately following in-office bleaching, the mean (SD) reduction in SGU for the 6% HP with Philips Zoom WhiteSpeed LED acceleration group was 6.9 (3.1), and 5.3 (2.5) for the 6% HP without LED acceleration group. This difference was statistically significant, p-value = 0.0001.
At Day 7 following in-office bleaching, the mean (SD) reduction in SGU for the 6% HP with Philips Zoom WhiteSpeed LED acceleration group was 4.4 (2.8), and 3.6 (2.5) for the 6% HP without LED acceleration group. This difference was statistically significant, p-value = 0.0089.
At Day 14 following in-office bleaching, and including three-dose use of Philips Zoom NiteWhite 16% carbamide peroxide application, the mean (SD) reduction in SGU for the 6% HP with Philips Zoom WhiteSpeed LED acceleration group was 6.9 (2.9), and 6.2 (2.6) for the 6% HP without LED acceleration group. This difference was statistically significant, p-value = 0.0148.
Safety
The incidence and severity of tooth sensitivity was reported as low following chairside
6% hydrogen peroxide treatment, with mild sensitivity reported during the home use period of Philips Zoom NiteWhite 16% carbamide peroxide.
Conclusions
In-office tooth bleaching with a 4x15-minute regimen of 6% hydrogen peroxide exceeded the ISO/TR 28642 defined ΔE* perceptibility thresholds (AT and PT) at all timepoints, compared to Baseline. By this definition, the addition of a three-dose treatment with 16% carbamide peroxide was similarly effective, and helped prevent color rebound.
In-office tooth bleaching with 6% hydrogen peroxide with Philips Zoom WhiteSpeed LED acceleration was statistically significantly superior to in-office bleaching with 6% hydrogen peroxide without LED acceleration, per VITA BleachedGuide 3D Master shade matching assessment at all study time points: Immediately, Day 7 and Day 14 following treatment.
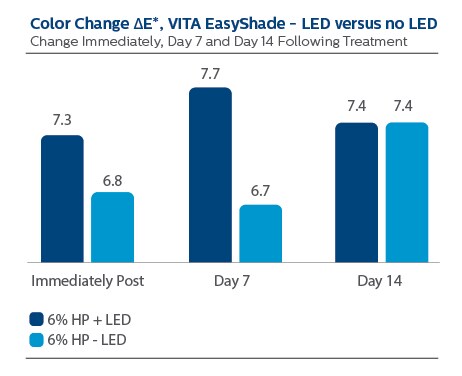
There was no statistical differentiation discerned between treatment groups per VITA EasyShade ΔE* measurement, at any time point.
© 2022 Koninklijke Philips N.V. (KPNV). All rights reserved. PHILIPS and the Philips shield are trademarks of KPNV. SONICARE and the Sonicare logo are trademarks of KPNV.

