Developed with the aim to increase efficiency and collaboration in your clinical lab As the first and currently only digital pathology solution in the U.S. to be marketed for primary diagnostic use, the technology can aid pathologists to view and diagnose digital images of surgical pathology slides. Digital pathology aims to reduce pressure on pathology services by streamlining workflow and extending collaboration with the aim of increasing diagnostic confidence.
What does this mean for me?
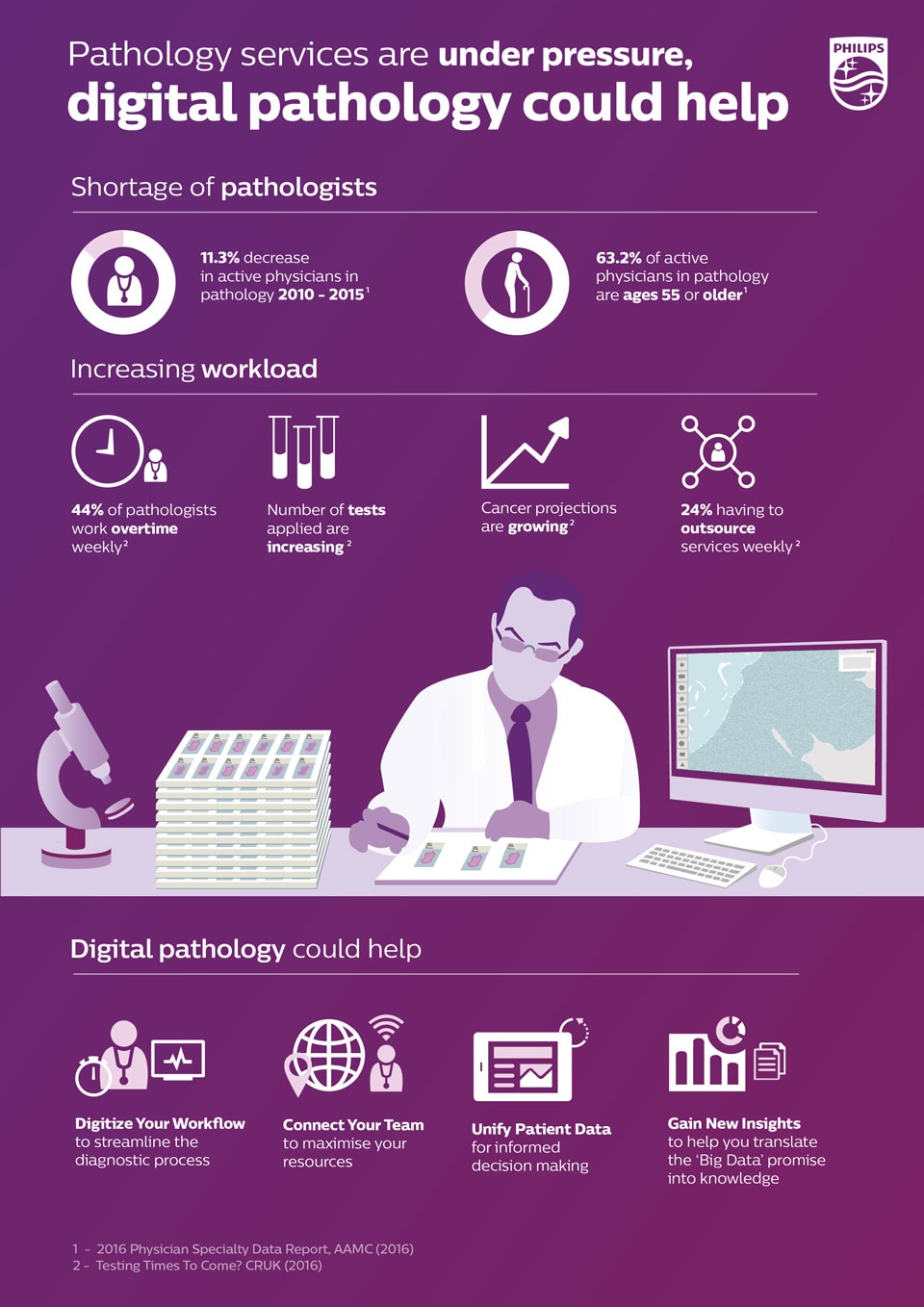
Pathology services are under pressure, digital pathology can help
Click on the button below to download the infographic “Pathology services are under pressure, digital pathology can help”.
Download white paper ‘Going Digital’

Alexi Baidoshvili: “At LabPON, we started using WSI in 2010 and discovered right away that we needed to have a fully digital diagnostics workflow to take advantage of all the benefits. To make the most of our experience, we want to share our insights with every department of pathology worldwide.
Philips IntelliSite Pathology Solution

Ultra Fast Scanner For routine use in high volume labs and integrated pathology networks.

Image Management System Open and scalable design offers optimal intergration into your workflow and IT infrastructure.

Image Management System viewer Easy access to images and tool/ resources to enable better informed decision-making.
In the European Union, the Philips IntelliSite Pathology Solution is CE Marked under the European Union's 'In Vitro Diagnostics Directive' for in vitro diagnostic use. In Canada, the Philips IntelliSite Pathology Solution is licensed by Health Canada for in vitro diagnostic use. In the United States, the Philips IntelliSite Pathology Solution can be used for in vitro diagnostic purposes. The Philips IntelliSite Pathology Solution is registered for in vitro diagnostic use in Singapore and Middle East.






