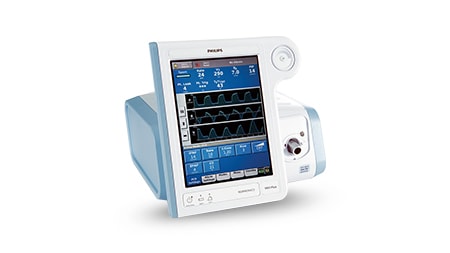
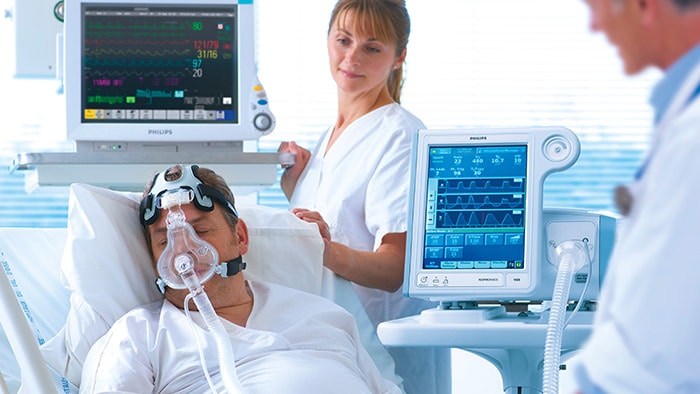
With over 40 years of expertise behind our ventilation portfolio, Philips has all the tools to help you with quality ventilatory care.
It’s more than just a ventilator, it’s the start of a partnership
We provide training and consultation to help clinicians expand their skillsets including how they handle and utilize healthcare equipment. Our ventilators solution has a complete set of patient-focused components and data connectivity to give you the information you need across the care journey to help you make your clinical decisions.