Safety Signs
| Reference | | Name | Description | Note |
| IEC 60878 0632 | 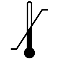 | Temperature limit | To identify the temperature limits, for example on transport packaging to indicate limits within which the package has to be kept and handled. The temperature values may be shown adjacent to the symbol | Indicates the temperature limits to which the medical device can be safely exposed. The upper and lowerlimits of temperature shall be indicated adjacent to the upper and lower horizontal lines. |
| IEC 60878 2620 |  | Humidity limitation | To indicate the acceptable upper and lower limits of relative humidity for transport and storage | |
| IEC 60878 2621 |  | Atmospheric pressure limitation | To indicate the acceptable upper and lower limits of atmospheric pressure for transport and storage | |
| IEC 60878 2497 |  | Date of manufacture | To indicate the date on which a product was manufactured. The date can be a year, year and month, or year, month, day. The date shall be placed adjacent to the symbol. The date may for example be given as follows: 1996-06-12 | Indicates the date when the medical device was manufactured. |
| IEC 60878 3082 | 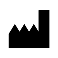 | Manufacturer | To identify the manufacturer of a product. This symbol shall be used filled in all applications to differentiate it from ISO 7000-2497 | This symbol shall be accompanied by the name and address of the manufacturer (i.e. the person placing the medical device on the market), adjacent to the symbol. |
| IEC 60878 2493 |  | Catalogue number | To identify the manufacturer’s catalogue number, for example on a medical device or the corresponding packaging. The catalogue number shall be placed adjacent to the symbol | |
| IEC 60878 2498 |  | Serial number | To identify the manufacturer’s serial number, for example on a medical device or its packaging. The serial number shall be placed adjacent to the symbol | |
| IEC 60878 1135 |  | General symbol for recovery/recyclable | To indicate that the marked item or its material is part of a recovery or recycling process | |
| IEC 60878 0533 | 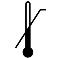 | Upper limit of temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed. The upper limit of temperature shall be indicated adjacent to the upper horizontal line. | |
| IEC 60878 0534 | 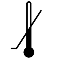 | Lower limit of temperature | Indicates the lower limit of temperature to which the medical device can be safely exposed. The lower limit of temperature shall be indicated adjacent to the lower horizontal line. | |
| IEC 60878 0615 | 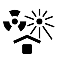 | Protect from heat and radioactive sources | Indicates a medical device that needs protection from heat and radioactive sources. | |
| IEC 60878 0624 |  | Keep away from sunlight | Indicates a medical device that needs protection from light sources or heat. | |
| IEC 60878 0626 | 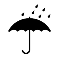 | Keep away from rain | Indicates a medical device that needs to be protected from moisture. | |
| IEC 60878 1051 | 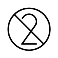 | Do not re-use | Indicates a medical device that is intended for one use or for use on a single patient during a single procedure. | |
| IEC 60878 2492 |  | Batch code | To identify the manufacturer's batch or lot code, for example on a medical device or the corresponding packaging. The code shall be placed adjacent to the symbol. | |
| IEC 60878 2499 |  | Sterile | Indicates a medical device that has been subjected to a sterilization process. | |
| IEC 60878 2500 |  | Sterilized using aseptic processing techniques | Indicates a medical device that has been manufactured using accepted aseptic techniques. | |
| IEC 60878 2501 |  | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. | |
| IEC 60878 2502 |  | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. | |
| IEC 60878 2503 |  | Sterilized using steam or dry heat | Indicates a medical device that has been sterilized using steam or dry heat. | |
| IEC 60878 2607 | 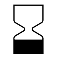 | Use by date | On packaging. To indicate that the device should not be used after the date accompanying the symbol, for example on a medical device or its packaging. The expiration date can be a year, year and month, or year, month, day. The date shall be shown adjacent to the symbol. The date may for example be given as follows: 1997-06-12. | IEC TR 60878 note: The date shall be expressed as in ISO 8601 as four digits for the year and, where appropriate, two digits for the month and two digits for the day. For some medical devices (e.g. IVDs), this date is only valid when the medical device is unopened. |
| IEC 60878 0621 |  | Fragile | Handle with care. Indicates a medical device that can be broken or damaged if not handled carefully. | |
| EN 50419 |  | Crossed-out wheeled bin | Marking of EEE (electrical and electronic equipment). Follow local requirements for proper disposal. | |
| FDA Final Rule - Rx Only |  | Prescription only | Prescription device. | |
| IEC 60878 2794 |  | Packaging unit | To indicate the number of pieces in the package. | |
| IEC 60878 2492 |  | Model number | To identify the model number or type number of a product. | |
| IEC TR 60878-5307 |  | Alarm Condition (High priority) | Indicates a high priority alarm condition on the device. | |
| IEC TR 60878-5307 |  | Alarm Condition | Indicates a medium or low priority alarm condition on the device. | |
| ISO 7010-W001 |  | General warning sign | Not applicable | |
| ISO 7000-3727 |  | Contains hazardous substances | To indicate that the medical device contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties | |

